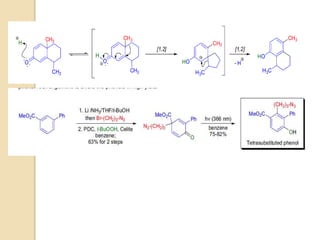
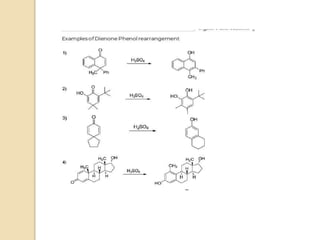
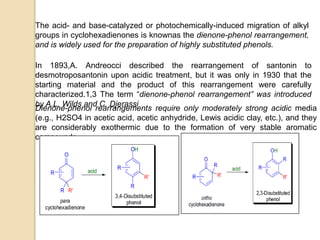
The document summarizes the dienone-phenol rearrangement, which is the acid- or base-catalyzed migration of alkyl groups in cyclohexadienones, resulting in highly substituted phenols. It was first described in 1893 for the rearrangement of santonin to desmotroposantonin under acidic conditions, but was more fully characterized in 1930. The rearrangement requires only moderately strong acids and is exothermic. It proceeds by a [1,3] sigmatropic migration of C-C bonds, which actually occurs through two subsequent [1,2] alkyl shifts. Depending on the migrating group, other rearrangements such as [1,2], [1,3], [

![Mechanism:
Most dienone-phenol rearrangements involve acid catalysis and the products appear to be the result of
sigmatropic [1,3]-migrations of C-C bonds.
The [1,3]-alkyl migrations are actually the result of two subsequent [1,2]-alkyl shifts as was demonstrated by
14C isotope labeling studies.
Depending on the nature of the migrating groups, other rearrangements such as [1,2], [1,3], [1,4], [1,5],
[3,3], [3,4], and [3,5] can also take place.
When the migrating group is benzyl, the products predominantly arise from [1,5]-migrations, and the rate of
these rearrangements is several
orders of magnitude greater than for simple alkyl groups. If the migrating group is allyl, crotyl, or propargyl,
then the main course of the rearrangement takes place via [3,3]-shifts rather than [1,2]-shifts.
The scheme below depicts the mechanism of the acid-catalyzed rearrangement of p-cyclohexadienone to the
corresponding 3,4-disubstituted phenol as well as the rearrangement of a bicyclic dienone via two subsequent
[1,2]-shifts.](https://image.slidesharecdn.com/dienonephenolrearragment-210521101204/85/Dienone-phenol-rearragment-3-320.jpg)