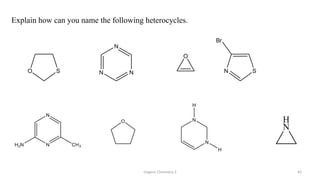
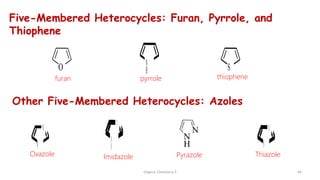
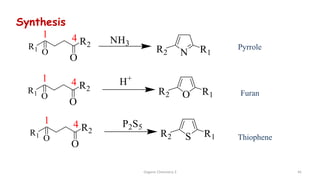
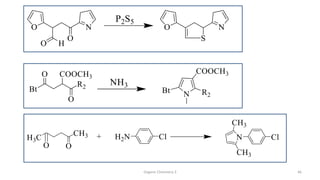
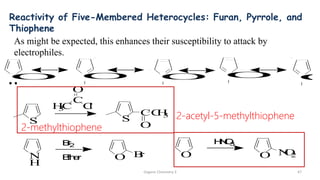
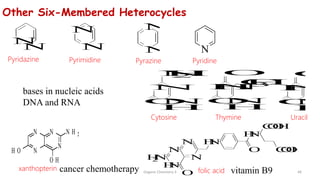
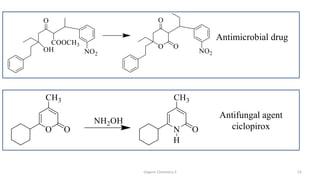
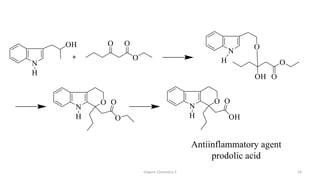
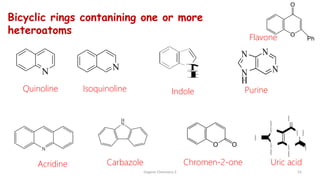
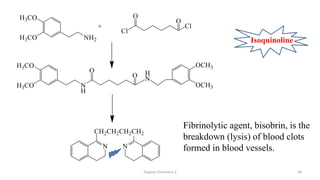
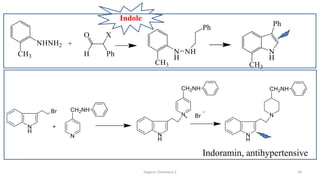
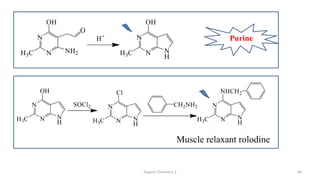
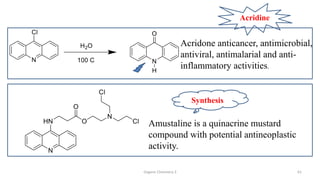
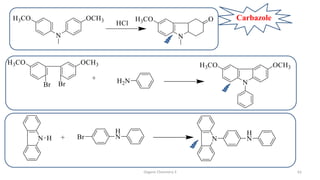
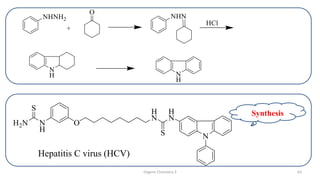
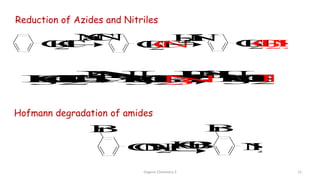
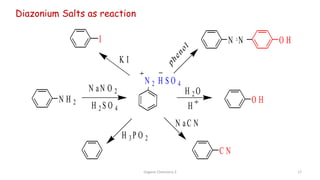
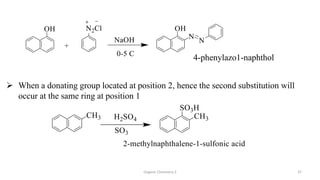
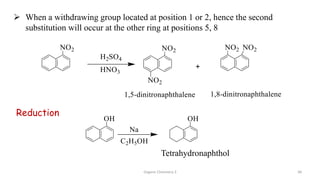
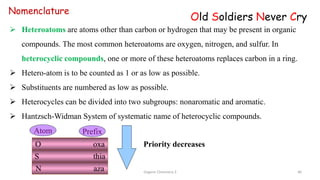
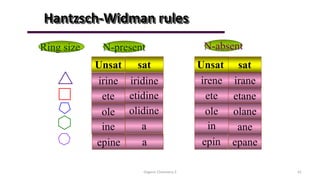
1. The document is from the Department of Pharmacy at the College of Medical Sciences in Yemen and discusses organic chemistry topics related to amines.
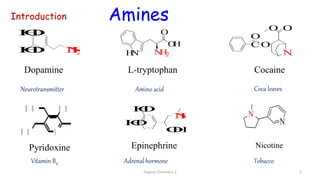
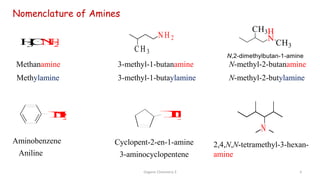
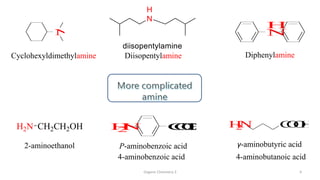
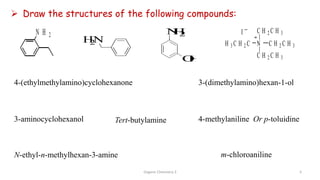
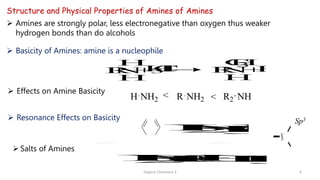
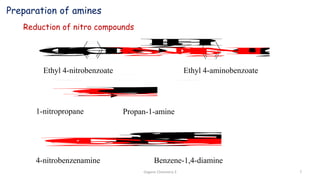
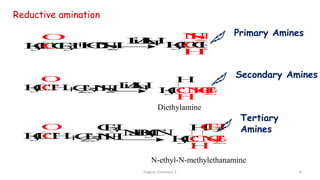
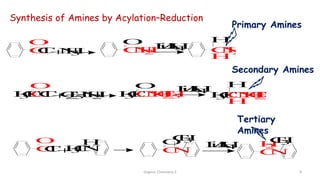
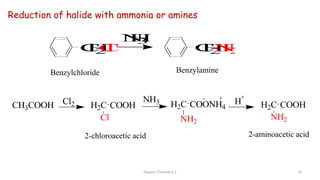
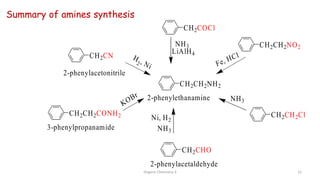
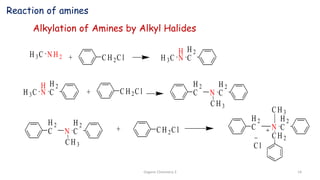
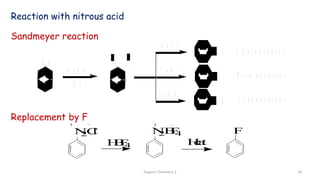
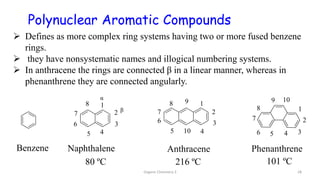
2. Key topics covered include the nomenclature, structure, properties, synthesis, and reactions of amines. Common amine synthesis methods like reduction of nitro compounds and reductive amination are described.
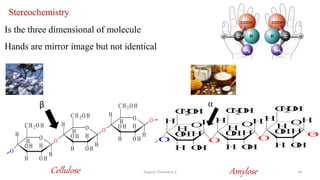
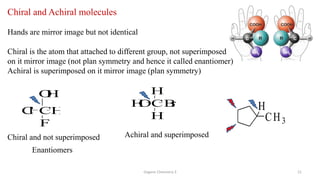
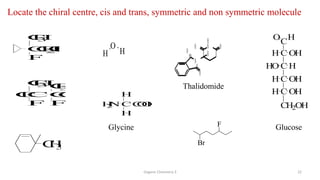
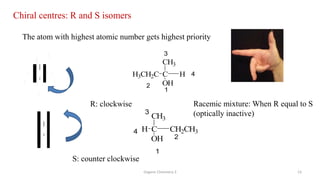
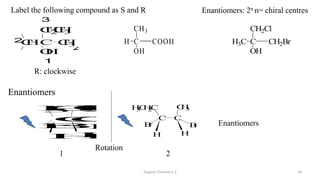
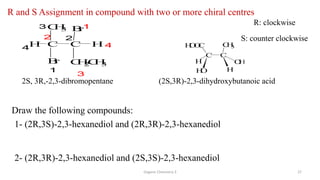
3. The document also discusses stereochemistry and isomerism related to amines, including chiral and achiral molecules, enantiomers, and diastereomers.







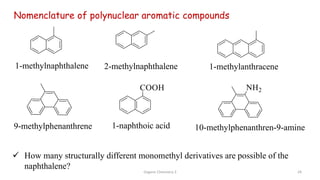
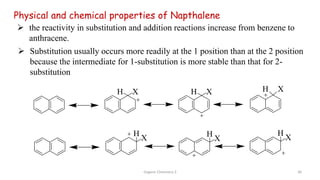
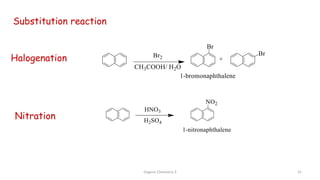
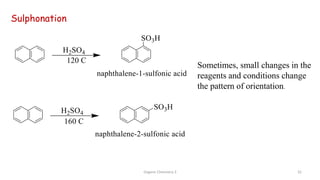

![Organic Chemistry 3 42
1H-Azole
pyrrole
1H-Azinane
piperidine
[1,3]-diazine
pyrimidine
1,2,5-Oxadiazole
1,2-oxazetidine
5-Amino-4-bromoisoxazole
2,3,4,5-Tetrahydroazine 1,2-Dihydroazine](https://image.slidesharecdn.com/organicchemistry3-240116164555-e38a8db5/85/organic-Chemistry-3-pptx-42-320.jpg)