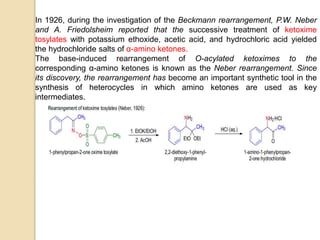
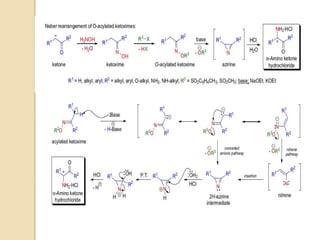
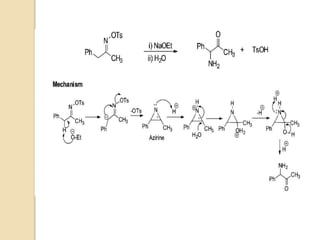
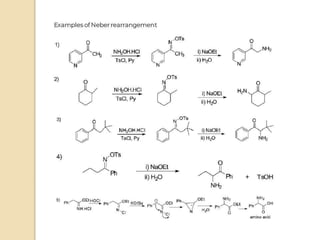
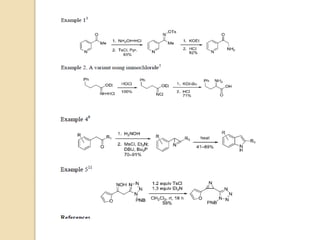
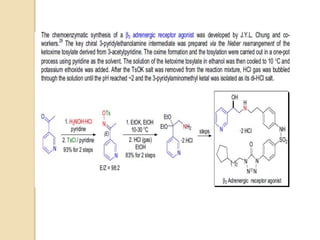
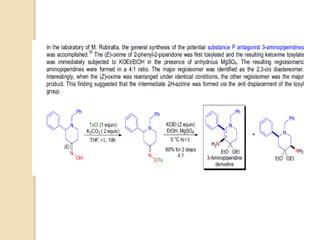
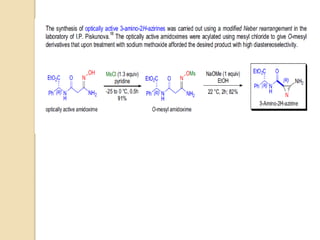
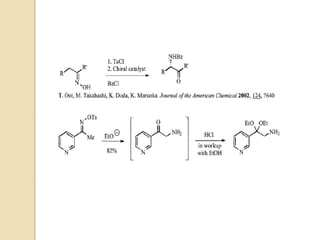
The Neber rearrangement is a base-induced rearrangement of O-acylated ketoximes to the corresponding α-amino ketones. It was discovered in 1926 by Neber and Friedolsheim during their investigation of the Beckmann rearrangement. The Neber rearrangement has since become an important synthetic tool for synthesizing heterocycles that use amino ketones as intermediates. It proceeds through an alkoxide-induced rearrangement of the O-acylated ketoxime to form an α-amino ketone product. There are some limitations in that aldoximes do not undergo the rearrangement and substrates generally require a methylene group in the α-position.