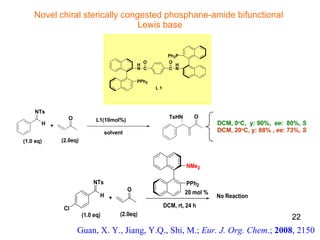
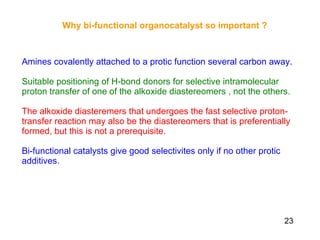
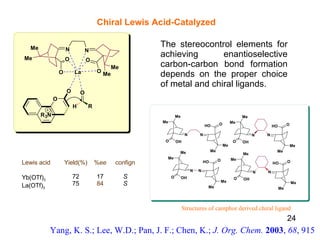
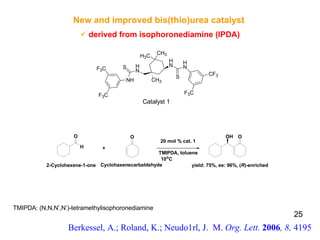
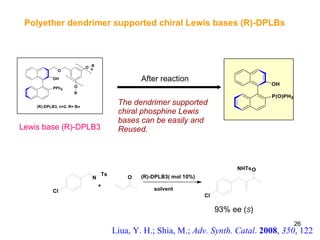
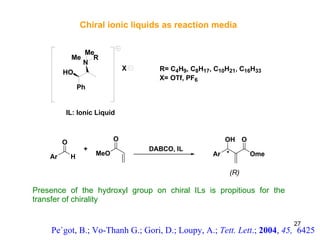
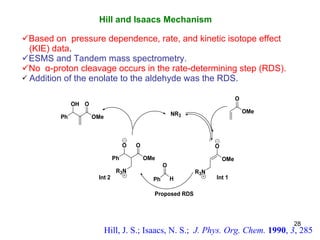
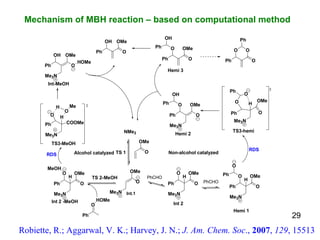
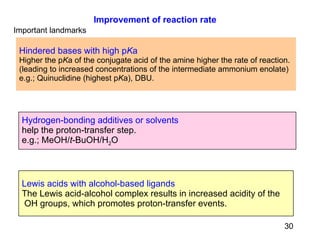
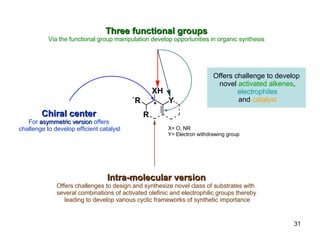
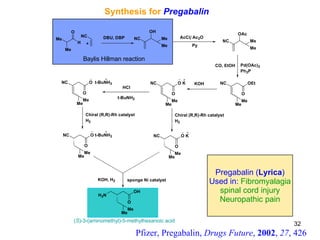
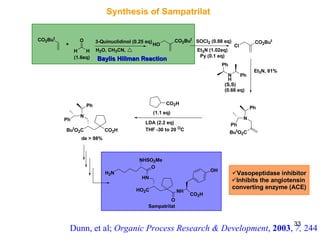
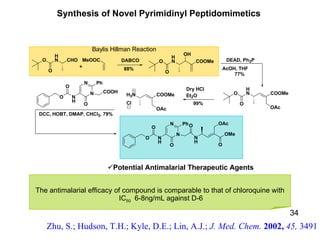
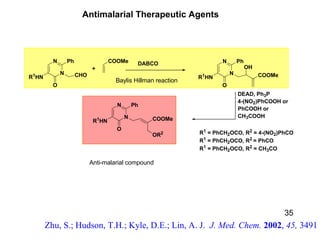
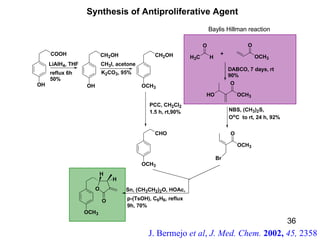
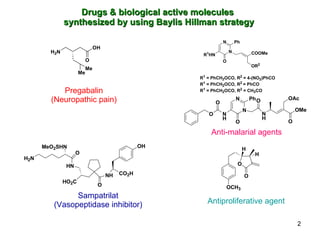
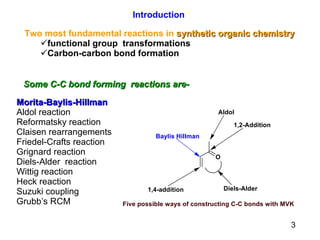
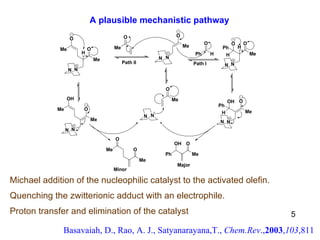
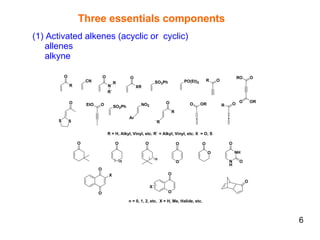
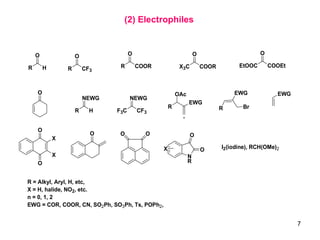
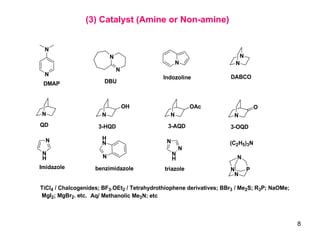
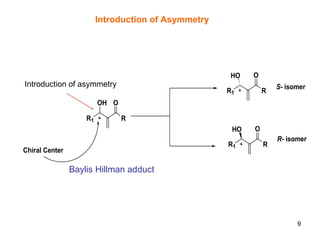
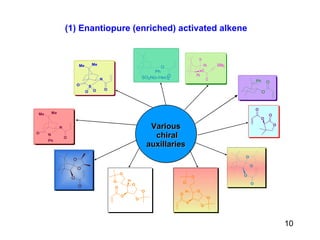
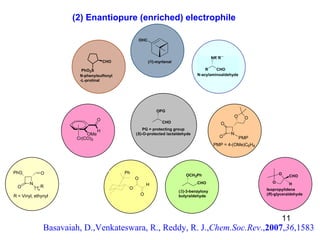
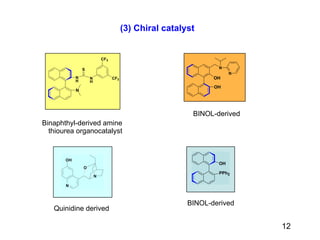
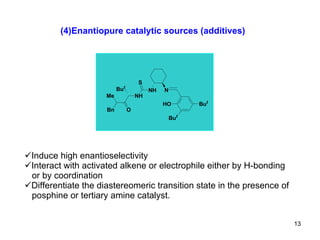
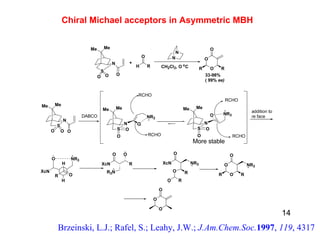
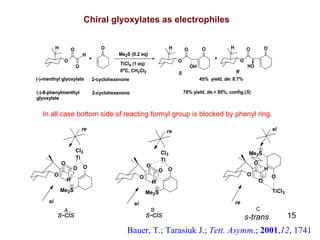
The document summarizes the Morita-Baylis-Hillman reaction, which forms carbon-carbon bonds between activated alkenes and electrophiles catalyzed by tertiary amines. It discusses introducing asymmetry, chiral catalysts used, and mechanisms. Examples are given of drugs synthesized using this reaction, including pregabalin and compounds with antiproliferative or antimalarial properties. In conclusion, the reaction enables easy carbon-carbon bond formation and synthesis of biologically active molecules.


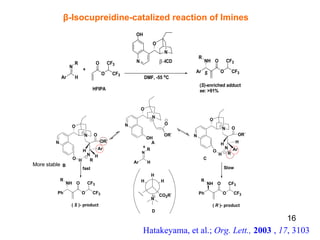
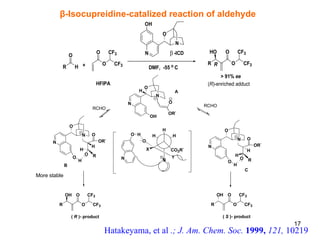
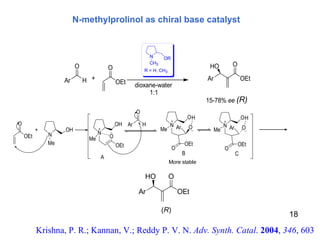
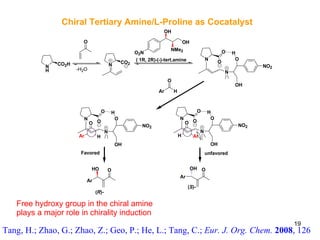
![Conformational lock in a Brønsted acid–Lewis base organocatalyst The acid–base functionalities help in substrate activation and fixing of the organocatalyst conformation to promote the reaction with high enantioselectivity . Mataui, K., Tanaka, K., Horii, A., Takizawa, S., Sasai, H.; Tett. Asym., 2006 , 17 , 578 1a : (S)-3-[4-(dimethylamino)pyridin-2-yl]BINOL 1b : (S )-3-[4-(dimethylamino)pyridin-3-yl]BINOL 1c : (S)-3-[3-(dimethylamino)pyridin-5-yl]BINOL 2a : (S)-3-(N-methyl-N-3-pyridinylaminomethyl)BINOL 2b : (S )-3-(N-methyl-N-2-pyridinylaminomethyl)BINOL 2c : (S)-3-(N-methyl-N-4-pyridinylaminomethyl)BINOL 21 β Proposed catalytic cycle for the bifunctional organocatalyst- mediated aza-MBH reaction Br Ø nsted acid unit Concept of chiral bifunctional organocatalyst 1a-b, 1c, 2a-c Novel chiral organocatalyst](https://image.slidesharecdn.com/lalit-kumar-1211633041277264-8/85/Lalit-Kumar-21-320.jpg)