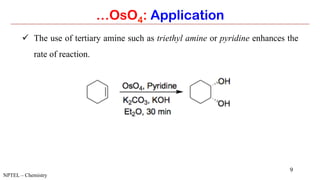
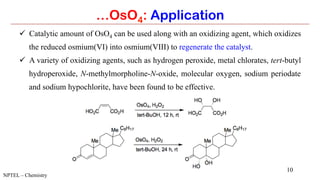
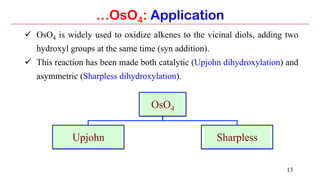
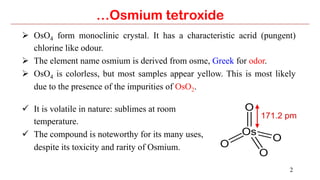
Osmium tetroxide (OsO4) is a volatile and toxic compound that is useful for the dihydroxylation of alkenes to form vicinal diols. It is also used in the Sharpless asymmetric dihydroxylation and Lemieux-Johnson oxidation reactions. Some key applications of OsO4 include the dihydroxylation, epoxidation, and oxidation of various organic compounds.





![7
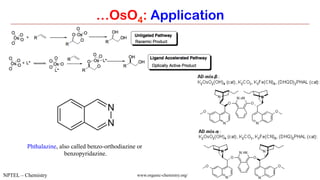
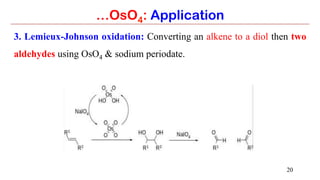
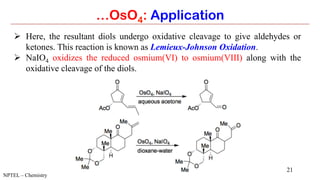
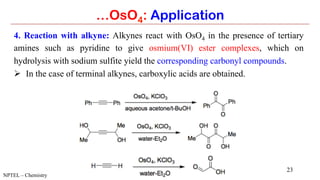
…OsO4: Application
1. Dihydroxylation of alkenes: Alkenes add to OsO4 to give diolate
species that hydrolyze to cis-diols via [3+2] cycloaddition. The net process
is called dihydroxylation. The reaction proceeds through an intermediate
osmate ester which rapidly hydrolyses to yield the vicinal diol.](https://image.slidesharecdn.com/11-210514082758/85/11-Oso4-7-320.jpg)