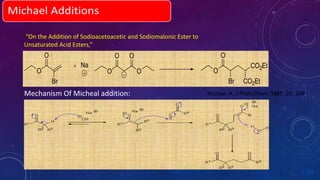
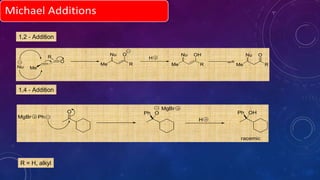
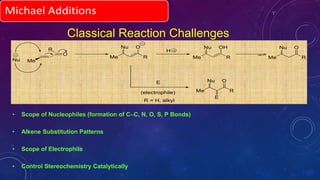
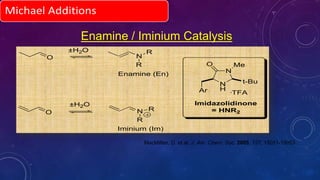
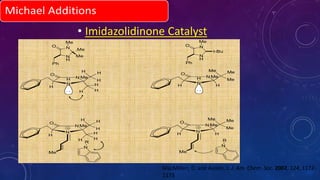
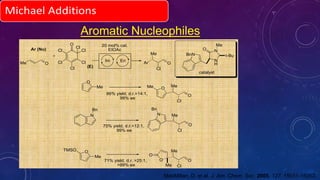
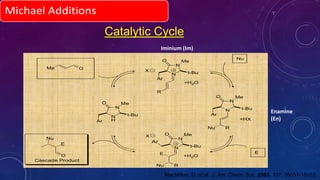
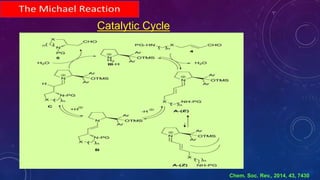
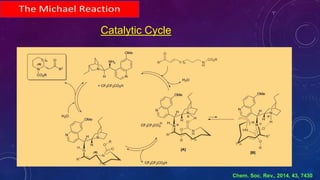
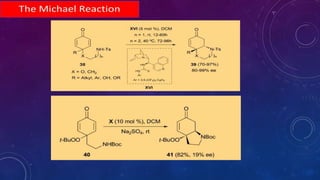
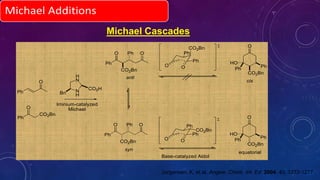
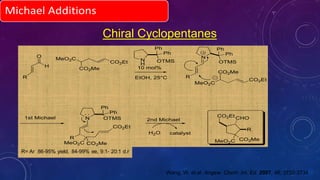
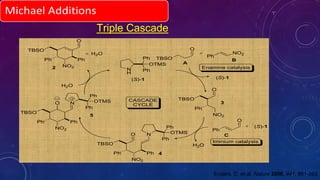
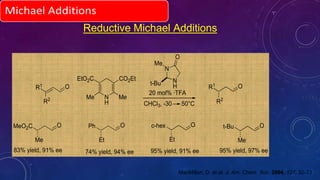
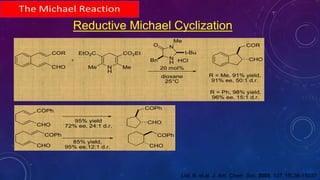
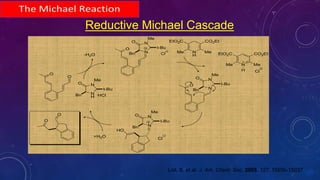
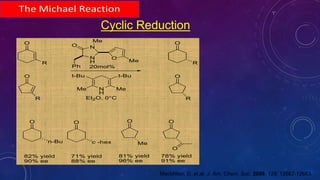
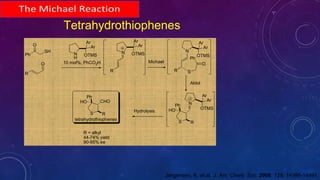
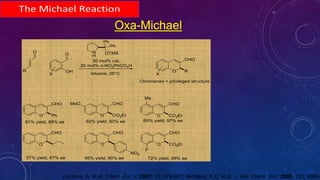
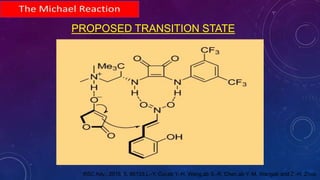
This document summarizes advancements in organocatalytic Michael additions. It describes the original Michael reaction and mechanism. Enamine/iminium catalysis is discussed, along with examples of aromatic and heteroatom nucleophiles in asymmetric Michael reactions. Michael cascades involving aldol reactions are also summarized. The document outlines reductive Michael additions and cascades catalyzed by iminium ions. Finally, it summarizes the development of asymmetric hetero-Michael reactions involving sulfur, oxygen, and nitrogen nucleophiles.