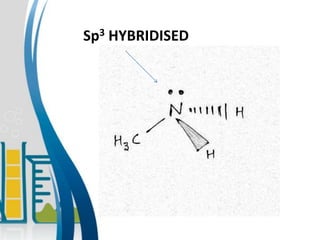
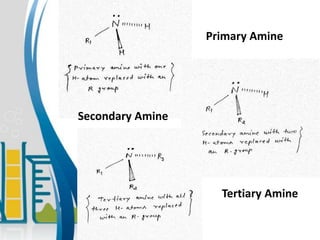
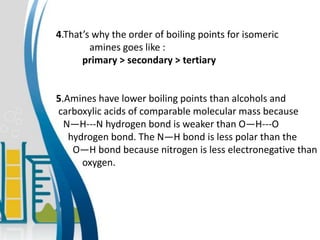
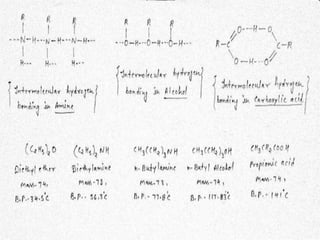
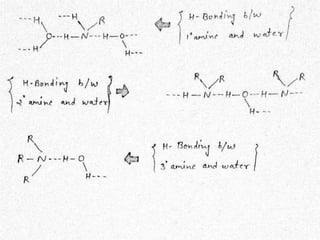
This document presents information about the structure and physical properties of amines. It was presented by Kuldeep Singh and Ujjwal Gola to their chemistry teacher, Dr. Vandana Kumari. The document discusses the sp3 hybridization and trigonal pyramidal structure of amines. It also describes the general structures of primary, secondary, and tertiary amines. The physical properties section explains that lower aliphatic amines are gases or liquids, amines have lower boiling points than comparable alcohols and carboxylic acids due to weaker hydrogen bonding, and solubility decreases with increasing alkyl group size.