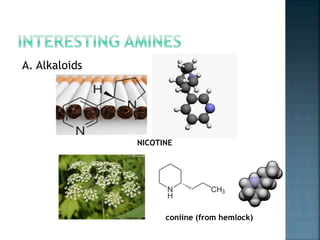
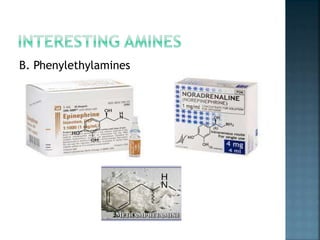
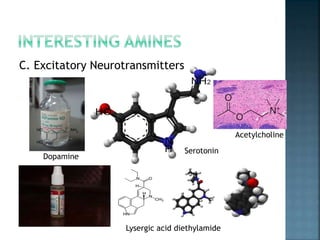
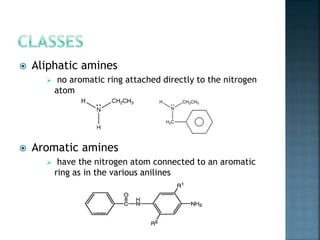
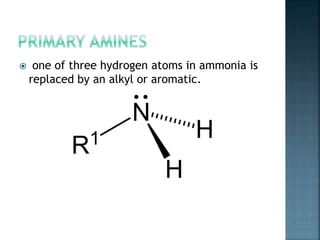
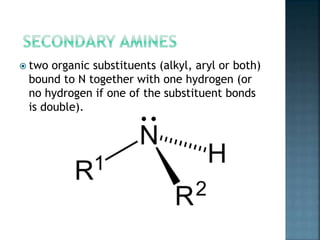
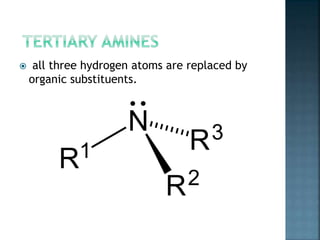
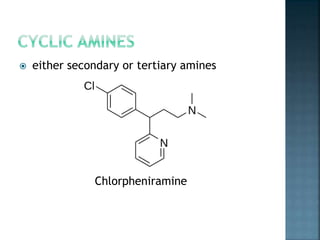
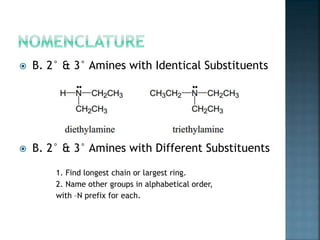
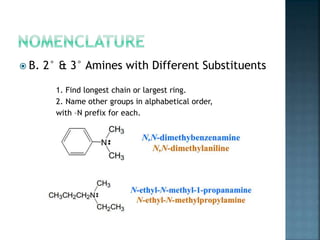
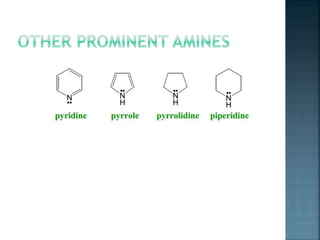
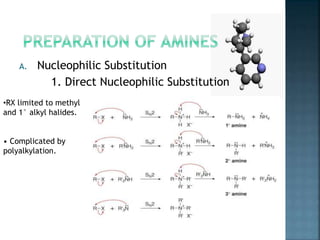
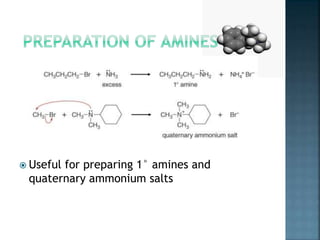
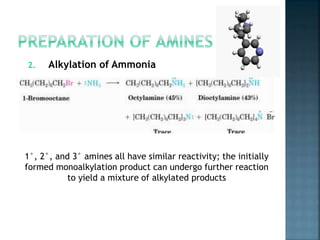
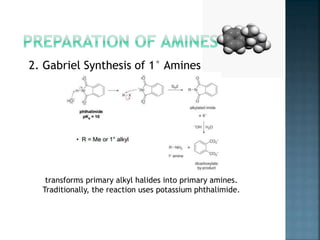
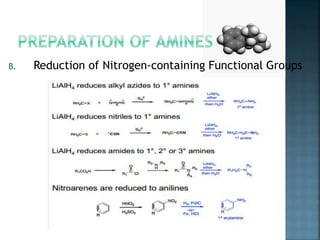
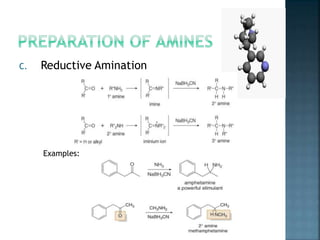
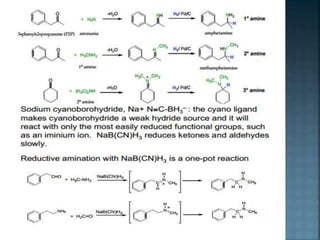
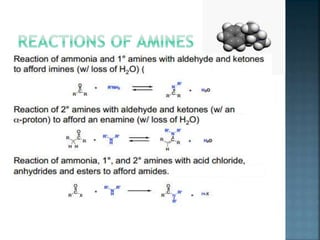
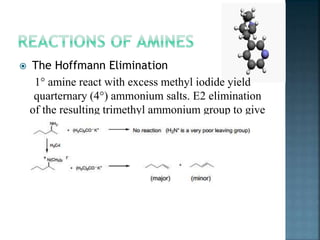
This document discusses amines, which are organic compounds derived from ammonia that contain a basic nitrogen atom. It describes different types of amines including primary, secondary, tertiary, aliphatic, aromatic, and cyclic amines. Methods for synthesizing amines are also summarized, including nucleophilic substitution reactions, reduction of nitrogen-containing functional groups, reductive amination, and the Hoffmann elimination reaction. Specific examples of important amines and their synthesis are provided.