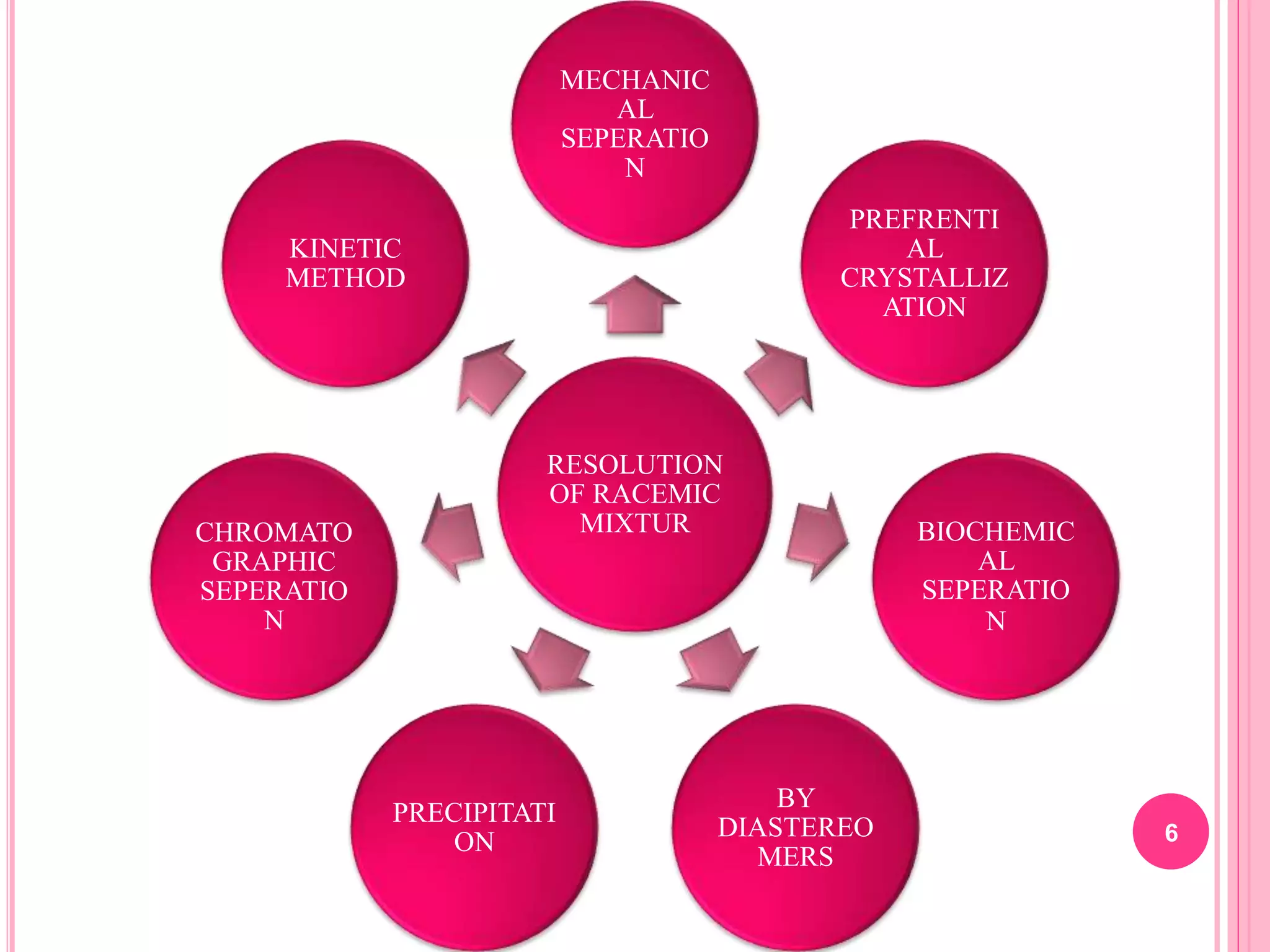
The document discusses various methods for resolving a racemic mixture into its pure enantiomers, including:
1) Mechanical separation, which separates crystal forms of enantiomers
2) Preferential crystallization, which seeds a solution with a pure enantiomer to induce crystallization
3) Biochemical separation using microorganisms that metabolize one enantiomer more quickly
4) Chromatographic separation exploiting differences in how enantiomers adsorb to a chiral support
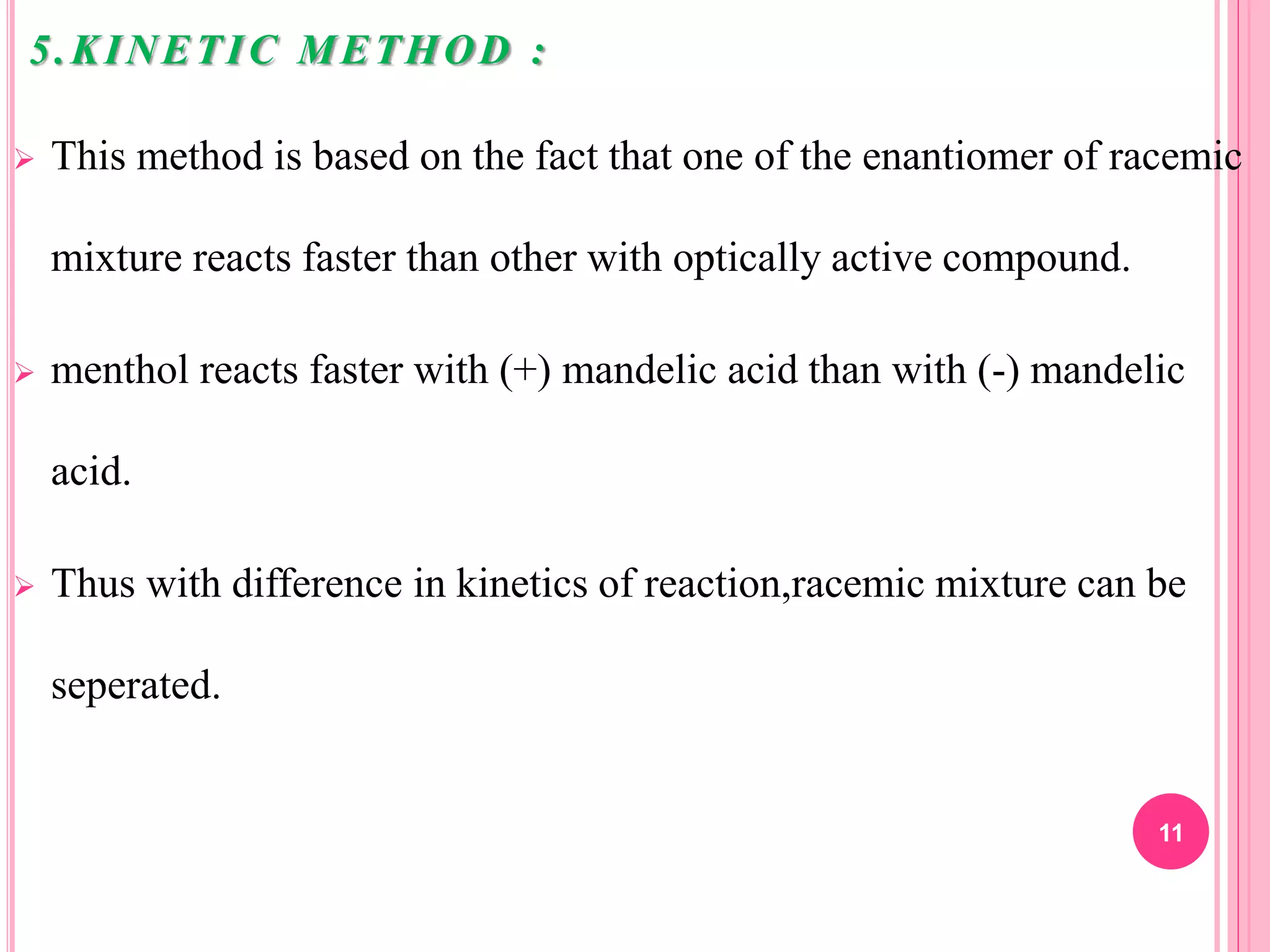
5) Kinetic methods using reactions that proceed at different rates for each enantiomer
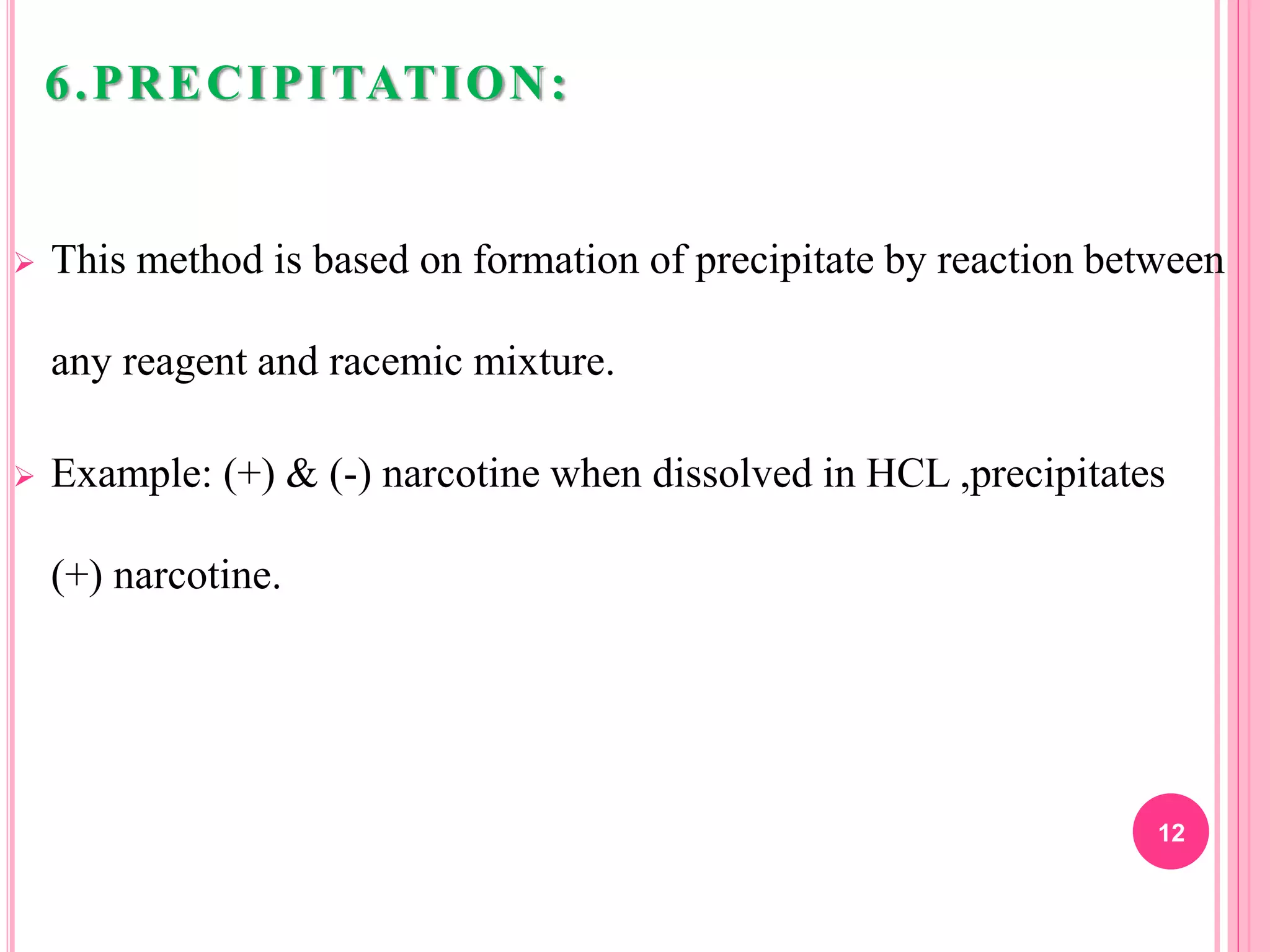
6) Precipitation of one enantiomer through reaction with a reagent
7) Formation of diastereomers with an opt