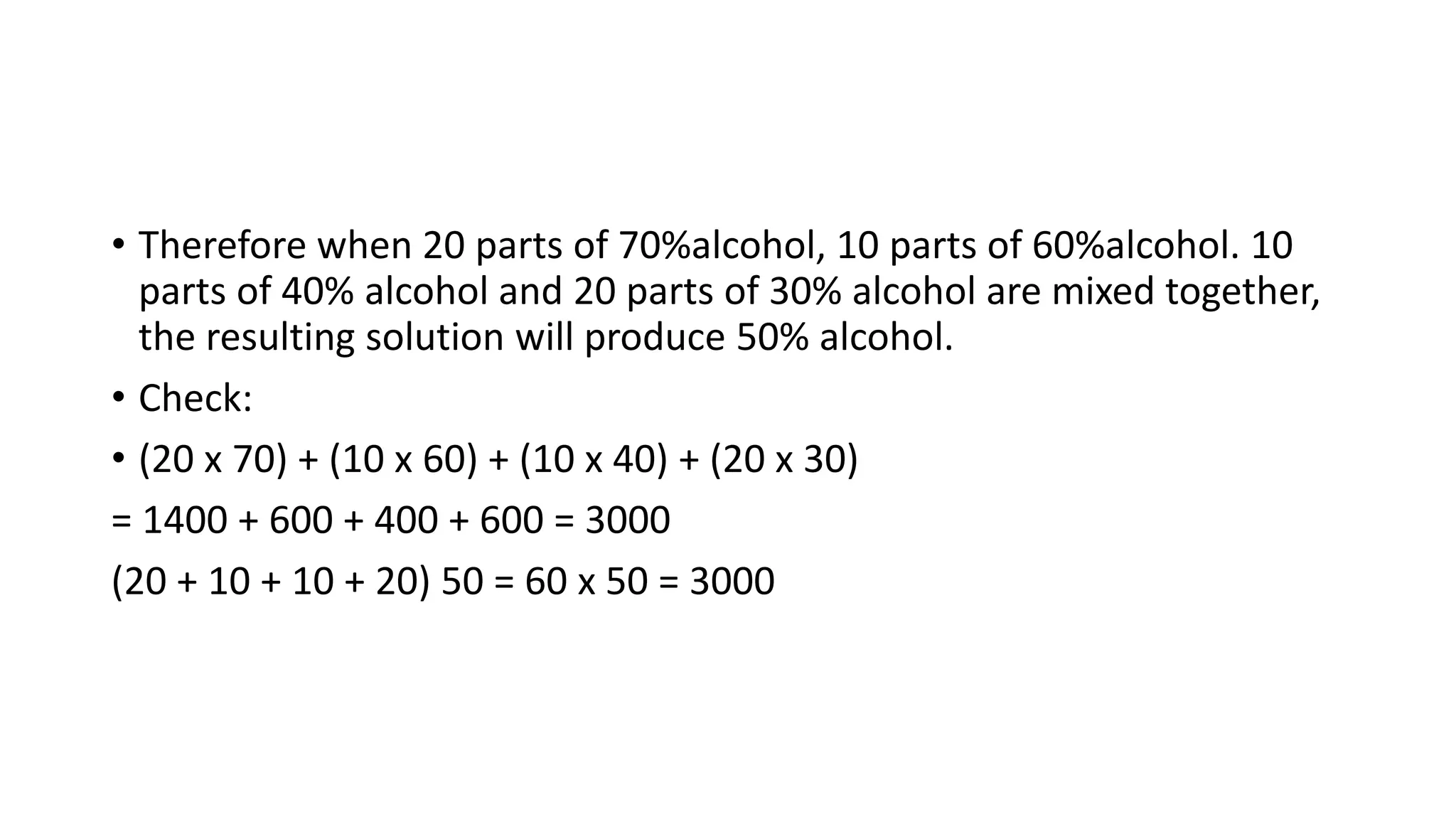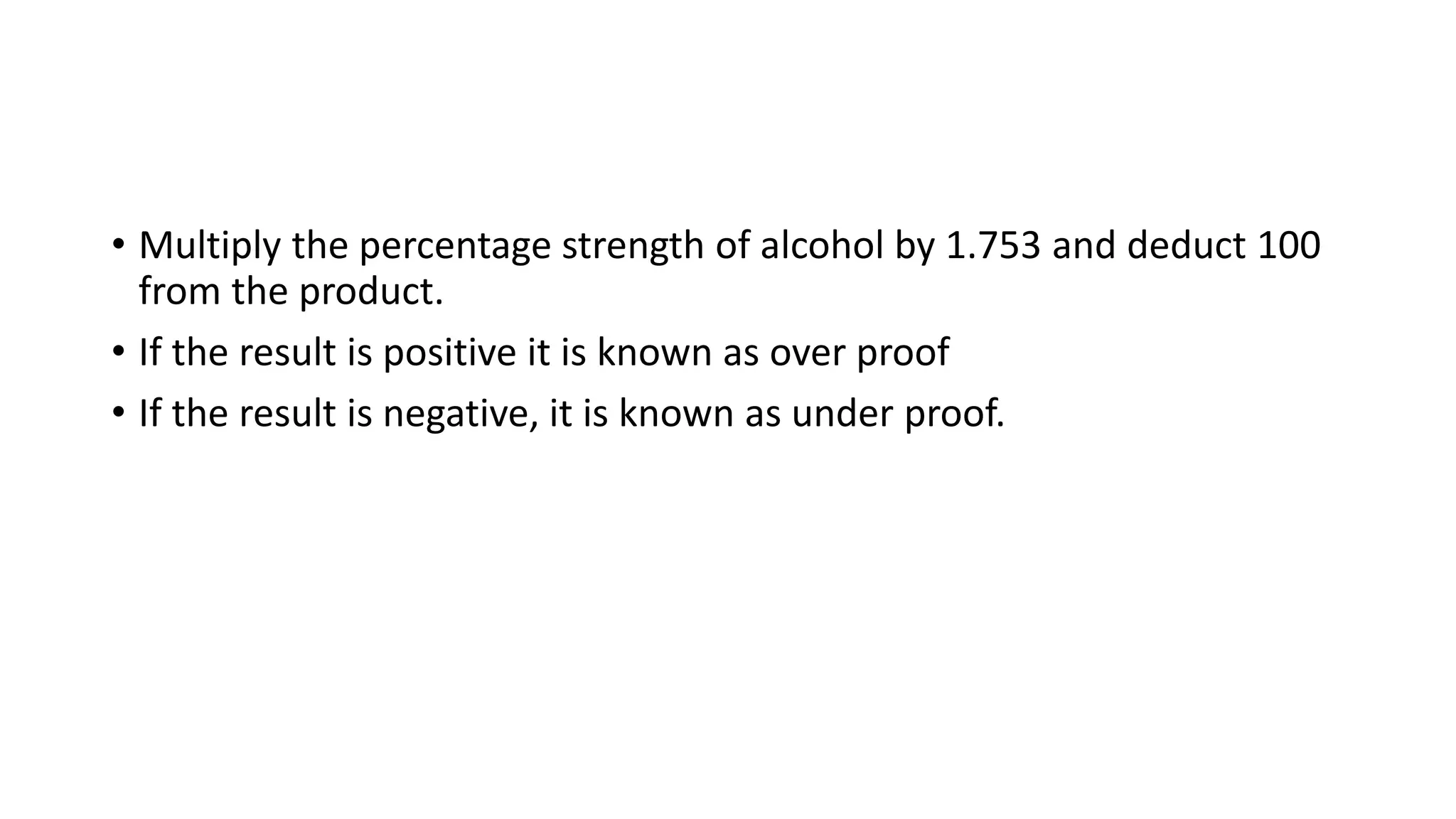This document discusses various pharmaceutical calculations related to dispensing medications. It covers:
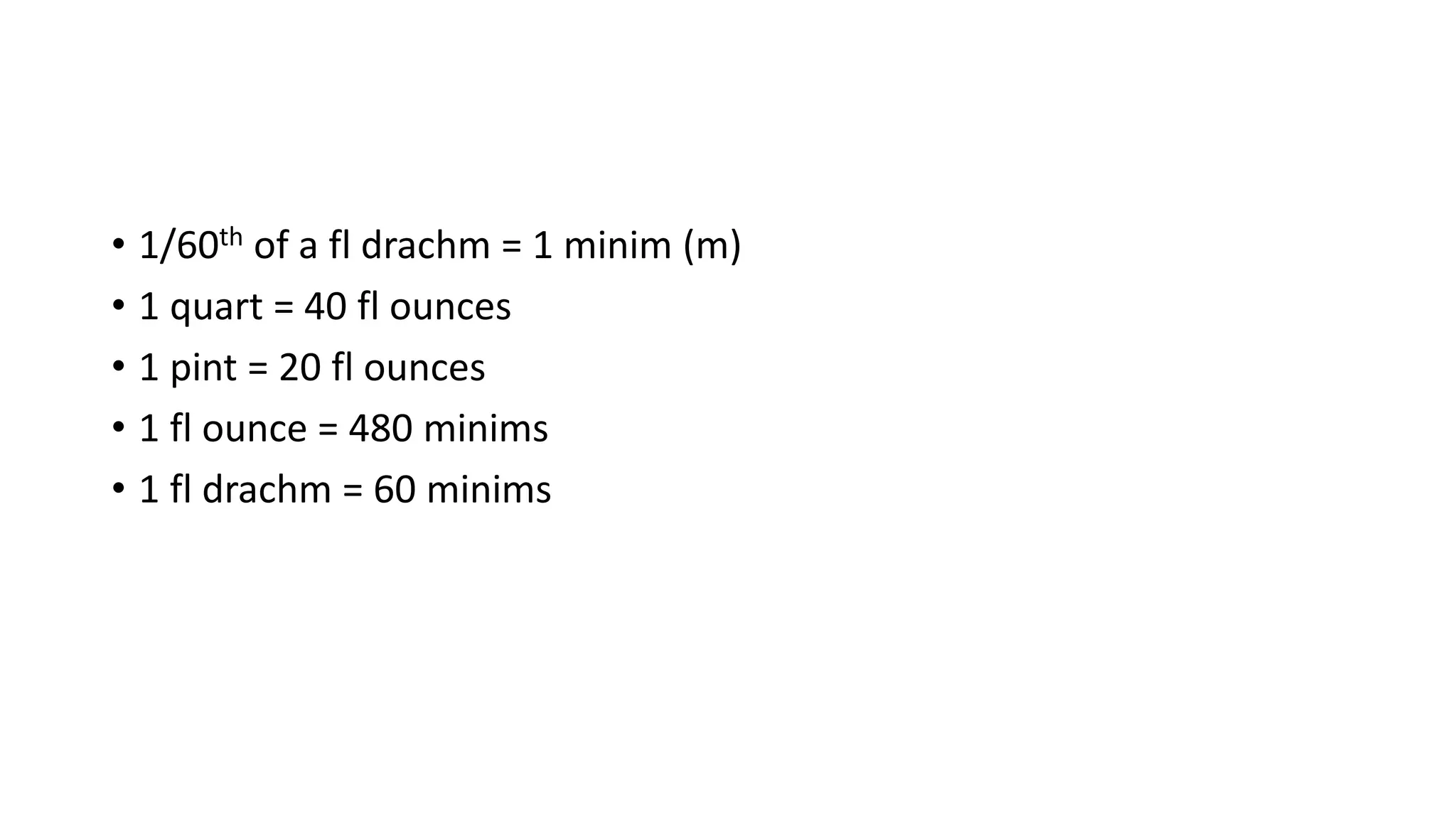
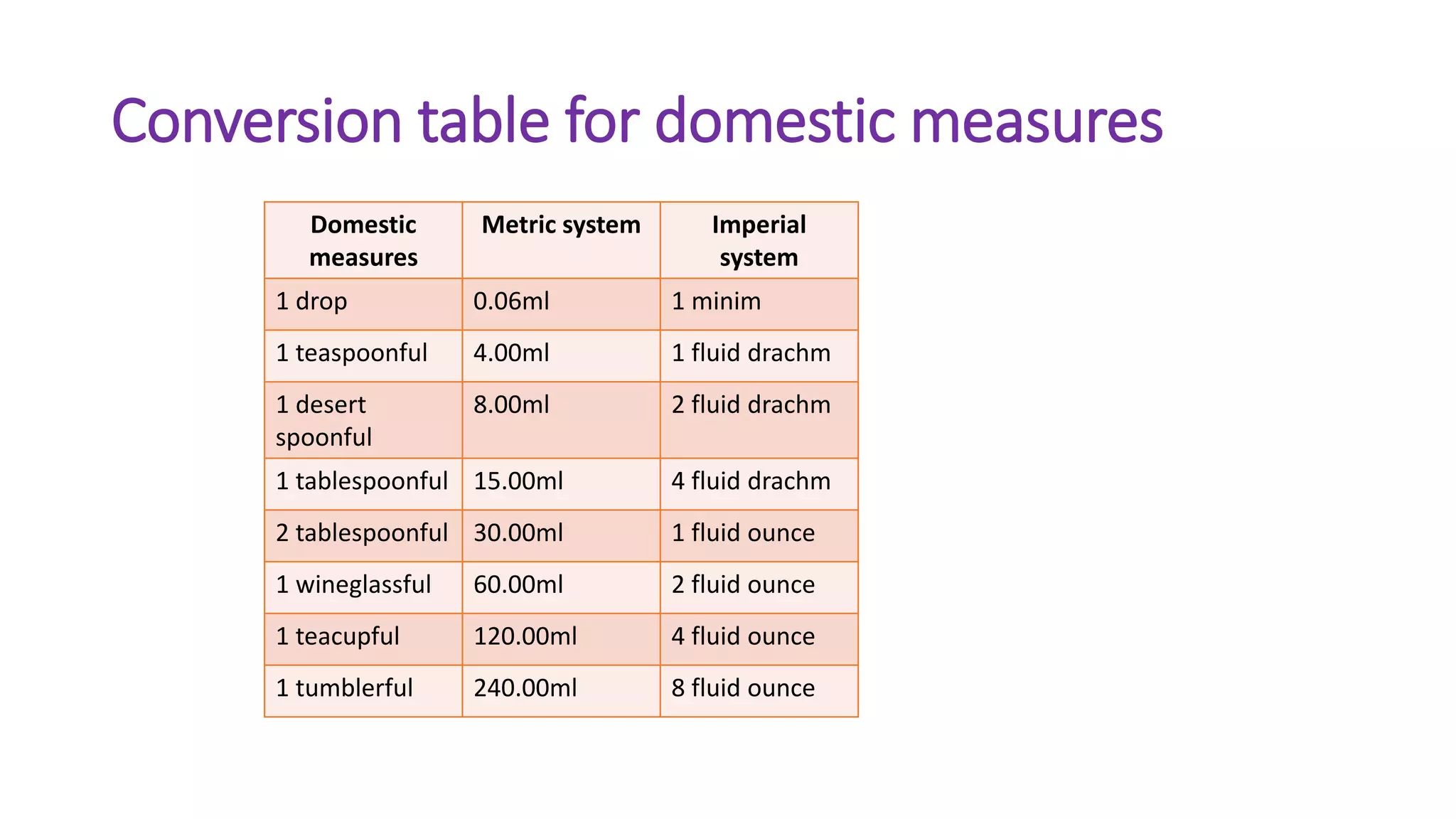
- Systems of weights and measures including avoirdupois, apothecaries, metric, and imperial.
- Calculations involving density, weight, and volume.
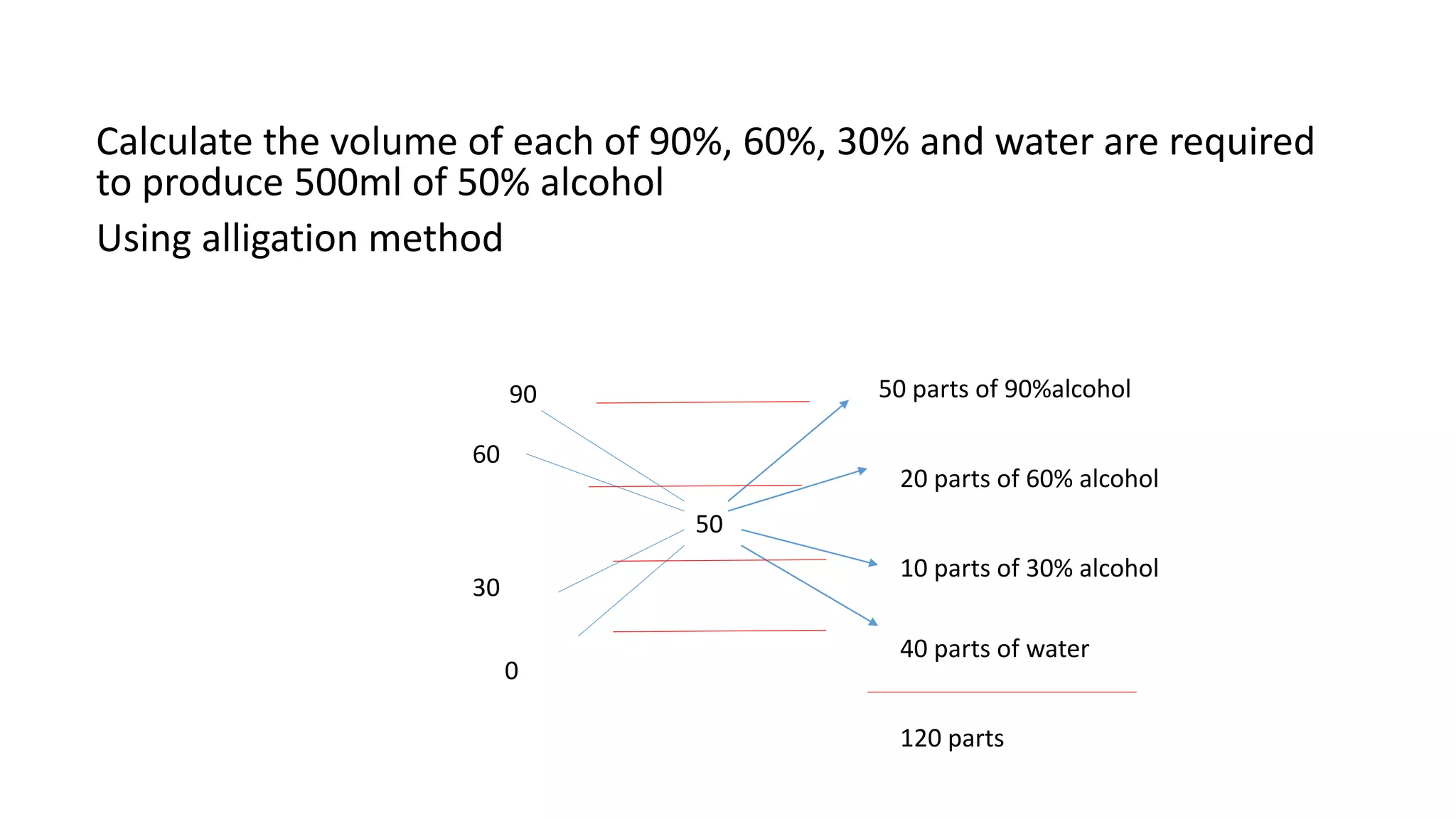
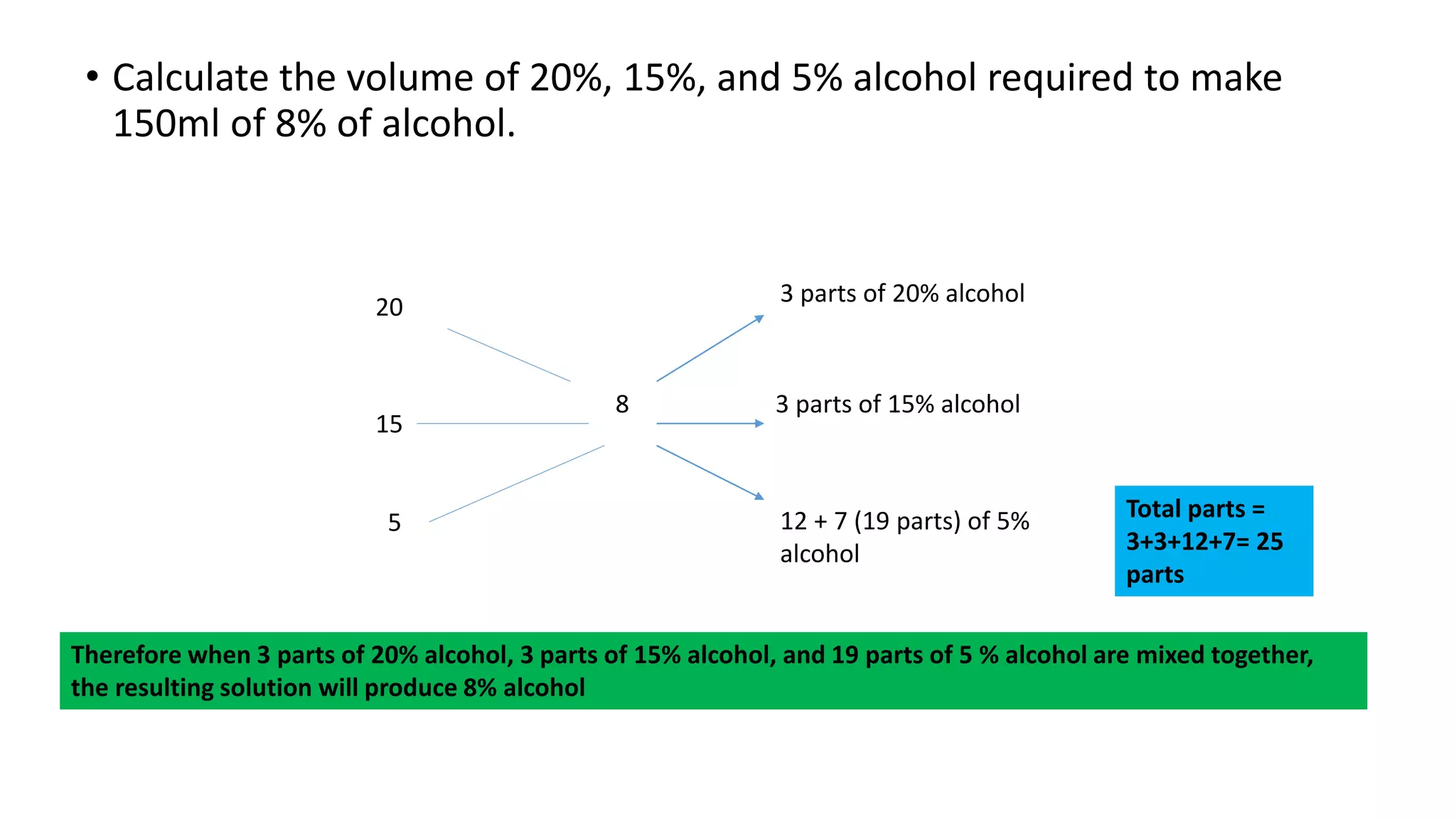
- Methods for calculating alcohol dilutions and mixtures to achieve a target concentration.
- Conversions between percentage solutions and proof spirit units used for excise purposes.
The document provides detailed examples and step-by-step workings for various calculation types pharmacists may encounter when dispensing prescriptions.