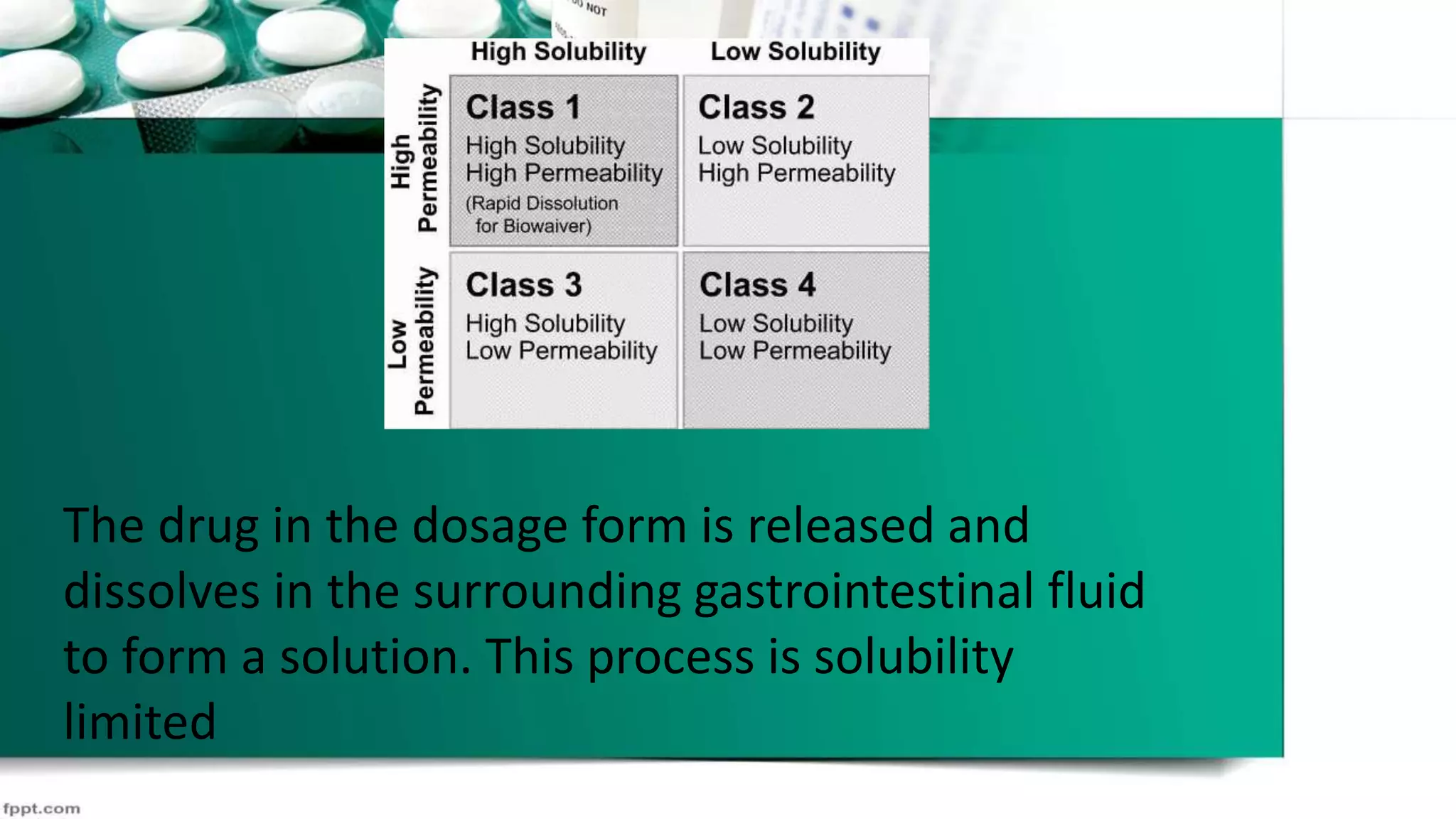
The document discusses the Biopharmaceutical Classification System (BCS), which classifies drug substances based on their aqueous solubility and intestinal permeability. The BCS takes into account three major factors that govern drug absorption from oral solid dosages: dissolution, solubility, and permeability. According to the BCS, drugs are classified into four classes based on being high or low solubility and permeability. Class I drugs have high solubility and permeability while Class IV drugs have low solubility and permeability. The classification can be used to predict drug absorption and bioavailability.