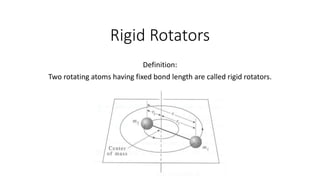
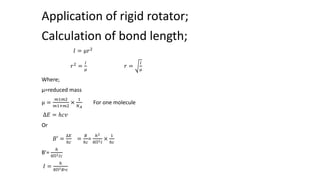
Rigid rotators are two rotating atoms with a fixed bond length that can be used to model diatomic molecules. They allow calculation of rotational energy classically using moment of inertia and quantum mechanically using the Schrodinger equation. Rotational energy is proportional to the rotational quantum number J and the rotational constant B, following the equation Ej = BJ(J+1). Transitions between rotational energy levels obey the selection rule that the change in J is ±1. Bond lengths can be calculated from the moment of inertia using the relation I = μr^2, where μ is the reduced mass.