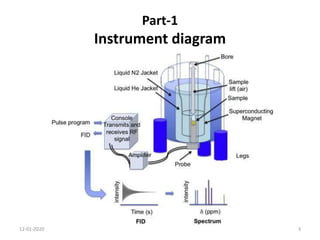
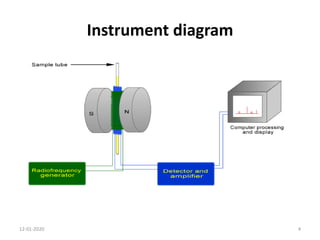
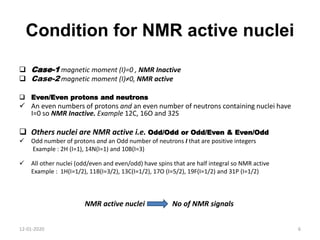
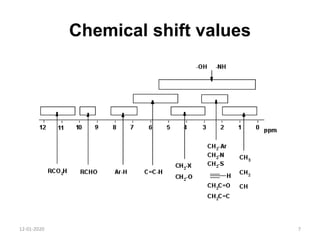
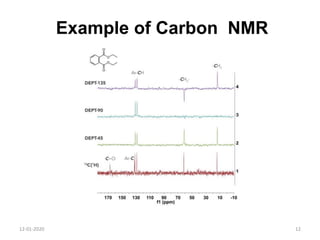
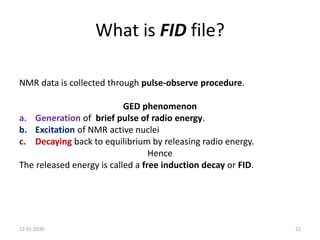
This document discusses nuclear magnetic resonance (NMR) spectroscopy, including instrumentation, analysis techniques, and sample preparation. It covers topics such as NMR-active nuclei, chemical shift values, solvent impurities, 1H and 13C NMR, peak assignment, integration, and using software to interpret results. Diagrams of NMR instrumentation are provided, as are examples of 1H and 13C NMR spectra and calculations of peak multiplicity. The use of deuterated solvents and sample preparation steps like vacuum drying and solubility testing are also outlined.