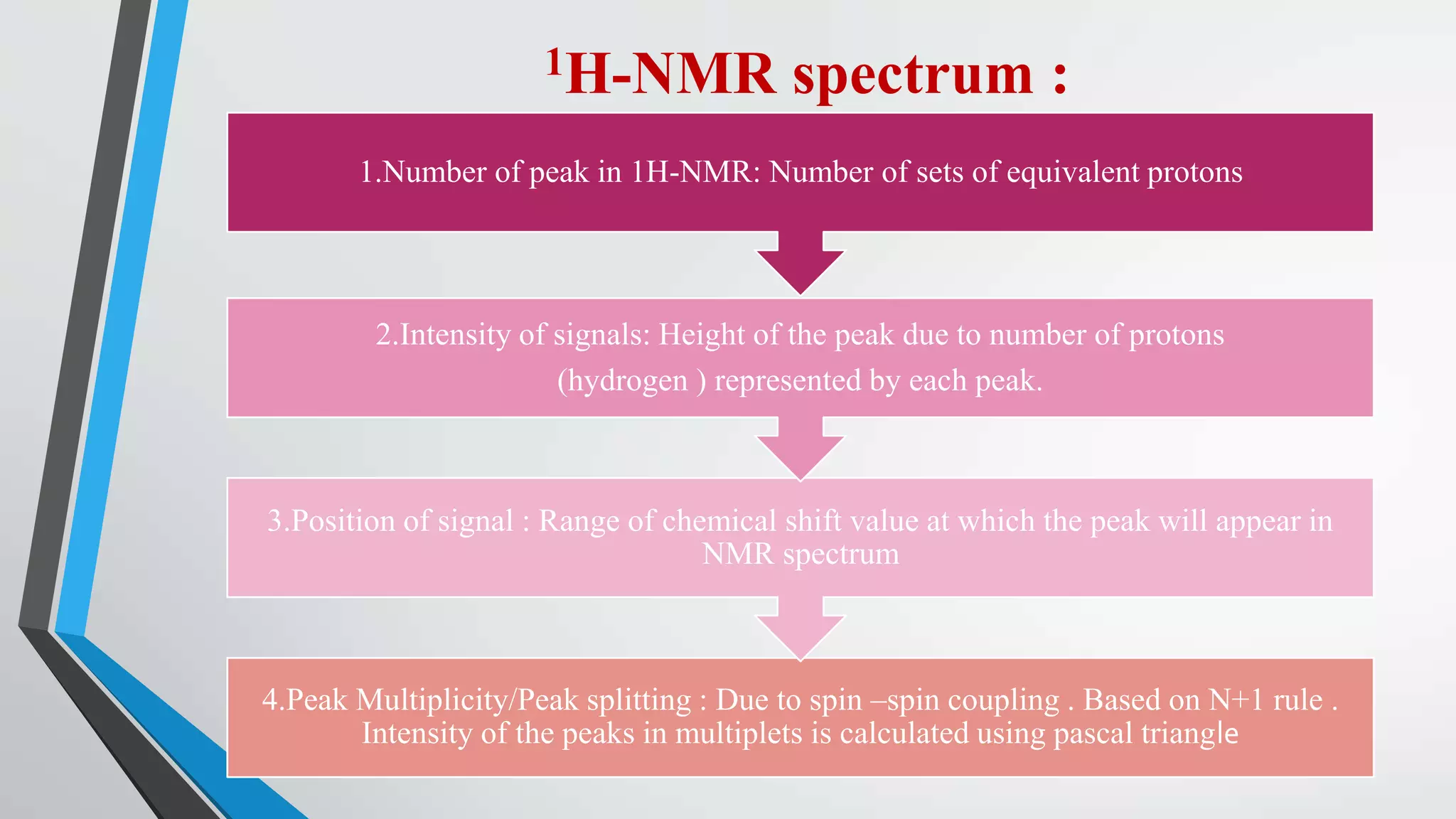
1) 1H NMR spectroscopy is a technique used to elucidate the structure of organic compounds by determining the number and environment of protons in a molecule.
2) The principle behind 1H NMR is that proton nuclei have a spin and when placed in a magnetic field will absorb radiofrequency energy, appearing as signals in the NMR spectrum.
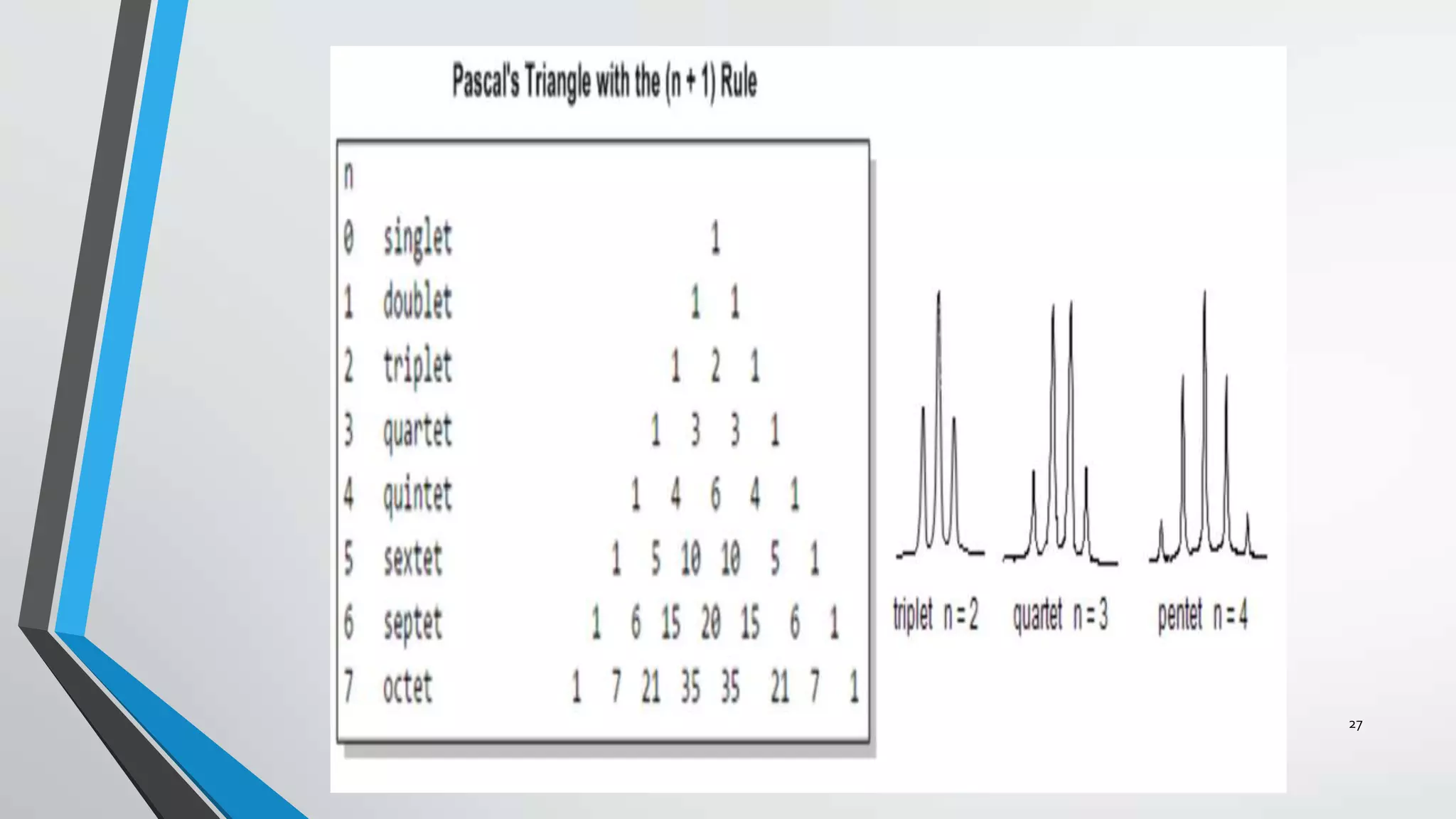
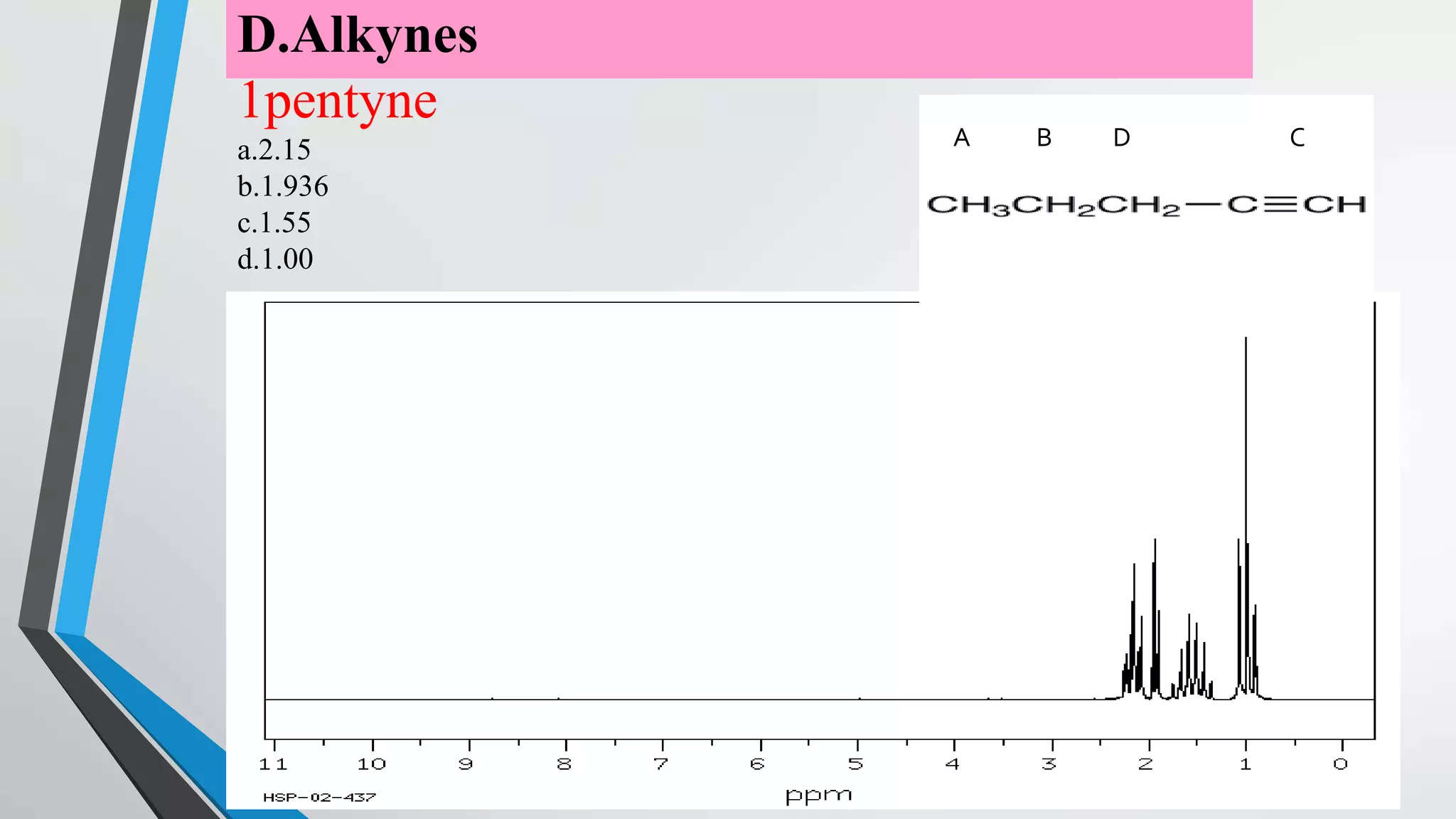
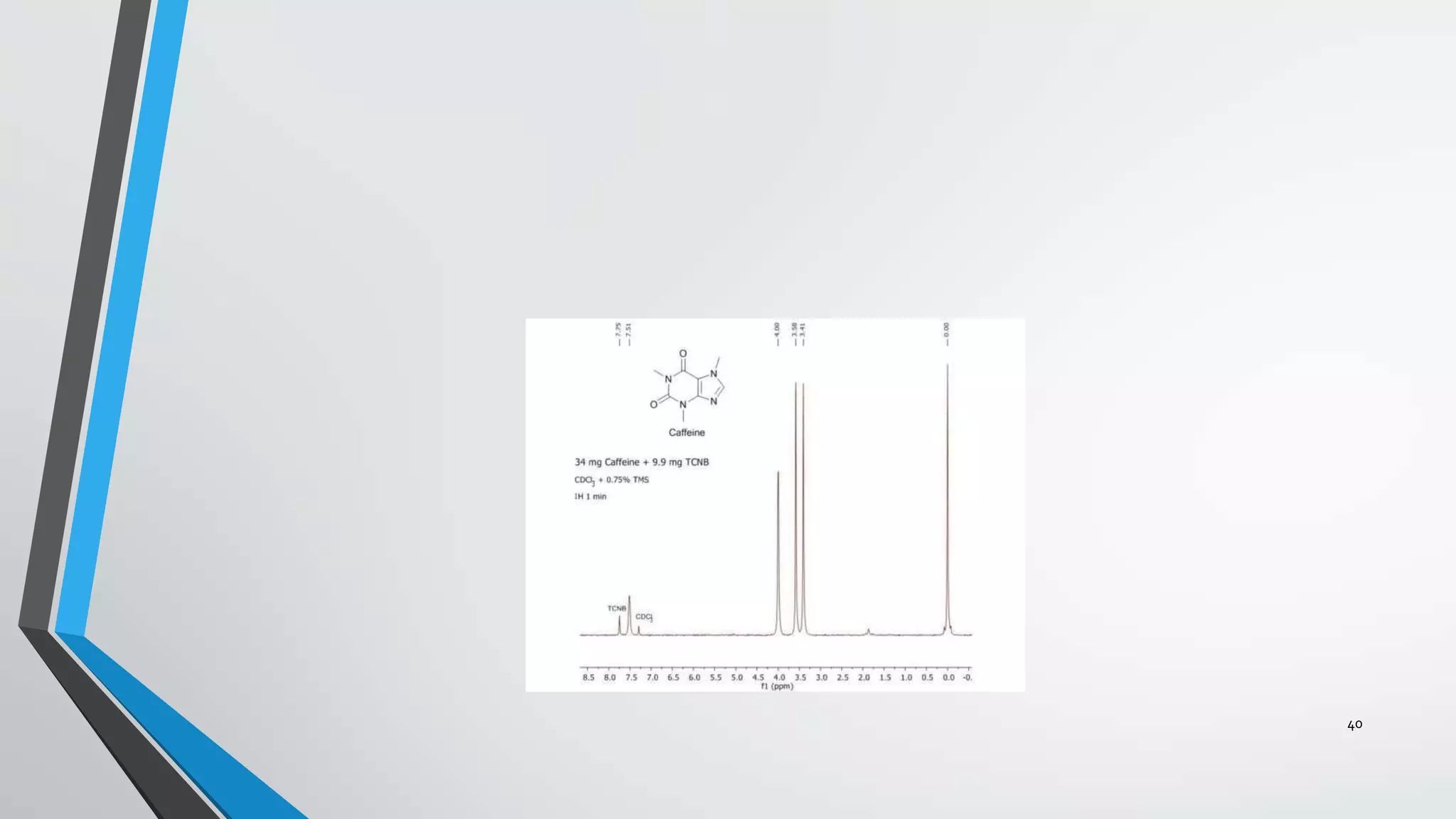
3) The chemical shift of 1H NMR signals provides information about the electronic environment and bonding of protons. Signals are referenced to TMS and appear in different regions of the spectrum.









































![APPLICATION
47
• Bacterial identification and metabolic studies
1H NMR spectroscopy has been used for bacterial identification and quantification and for metabolic
pathways studies. Several studies have been conducted for the diagnosis of the bacteria that cause urinary
tract infections (UTI).
• Antimicrobial susceptibility assays
The use of 1H NMR spectroscopy for antimicrobial susceptibly tests has been not highly studied despite its
powerful utility in this area of study [5]. Application of 1H NMR spectroscopy to antimicrobial
susceptibility studies was first carried out on
• Applications in bio fluids
In the last few years, 1H NMR has been used to directly analyse biofluids and to diagnose different
diseases directly from body fluids. In this sense, it has been applied to analyse human microbiota from
faeces and urine samples
• Other types of analyses
The combination of NMR spectroscopy, with the use of isotopically substituted molecules as tracers is a
well‐established protocol in microbiology.](https://image.slidesharecdn.com/1hnmrspectroscopynilam1-230809175425-7464cefe/75/Structural-elucidation-by-NMR-1HNMR-46-2048.jpg)

