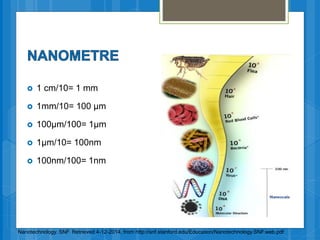
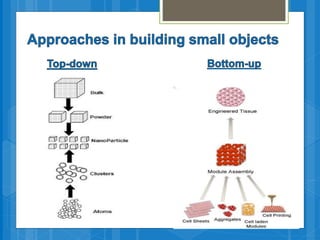
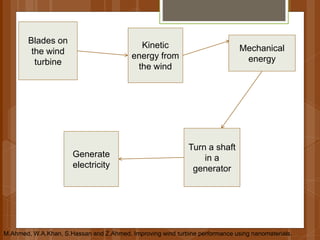
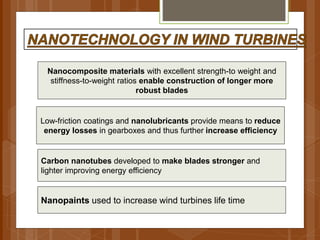
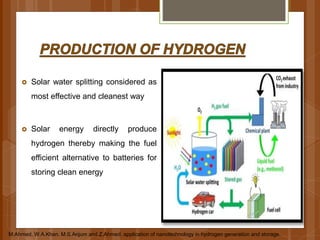
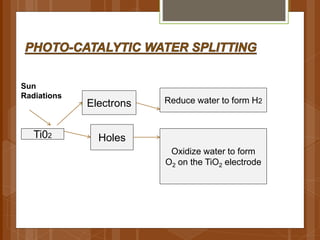
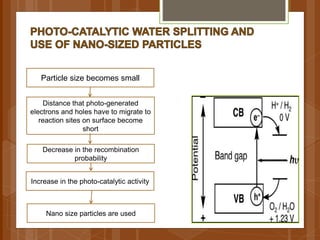
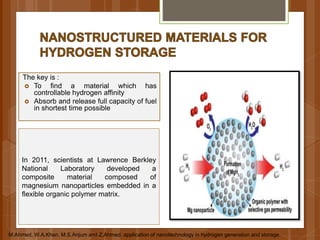
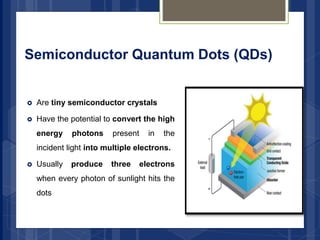
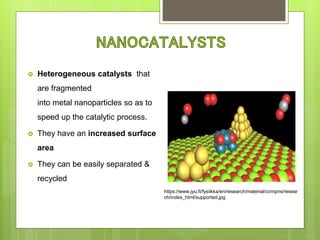
The document discusses various applications of nanotechnology in renewable energy and energy storage. It describes how nanomaterials and structures can be used to improve solar cells, batteries, fuel cells, hydrogen production and storage, and enhance the efficiency of technologies like wind turbines. For example, it mentions that nanoparticles or nanowires in solar cell electrodes can increase surface area and improve energy capture. Nanotechnology also aims to develop cheaper catalysts and better electrolyte materials to advance fuel cells.