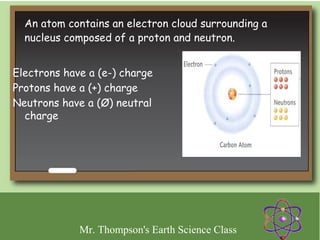
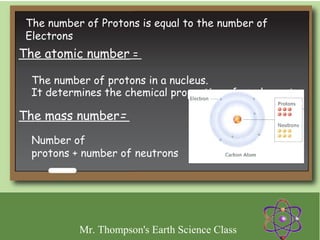
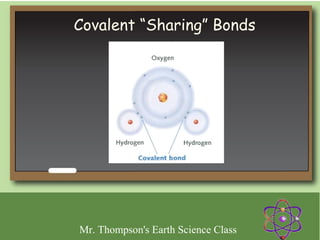
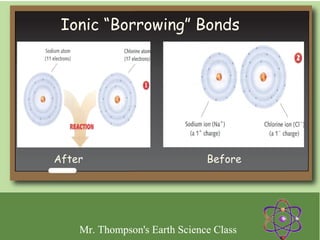
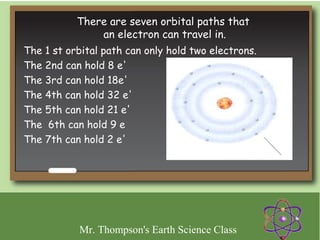
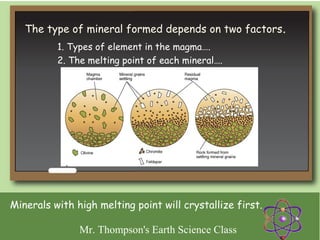
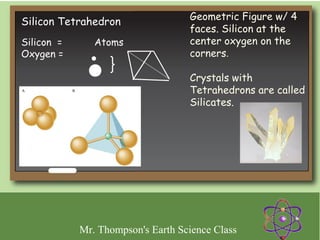
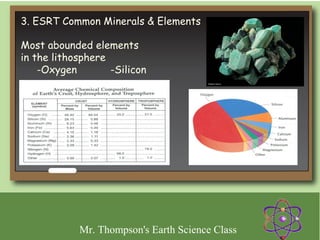
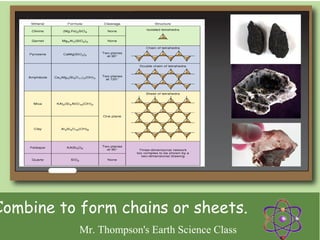
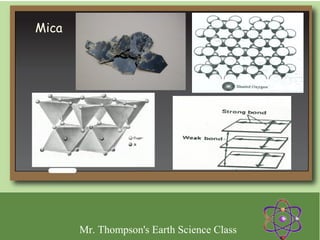
Minerals are naturally occurring inorganic solids with a defined crystal structure and chemical composition. They are composed of elements or compounds and are the building blocks of rocks. Minerals can be identified based on physical properties like hardness, crystal structure, color, and cleavage or chemical properties like acid reaction or magnetism, which are determined by the arrangement of atoms within the mineral.