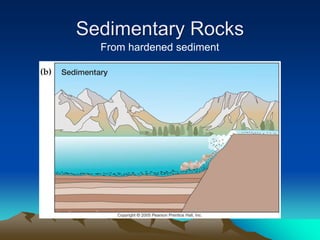
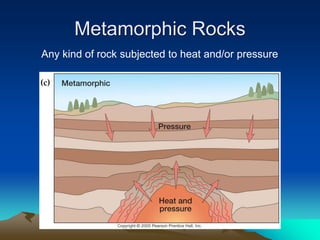
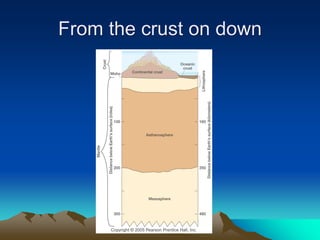
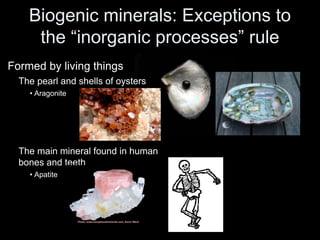
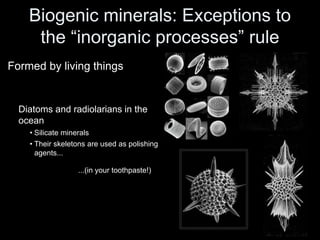
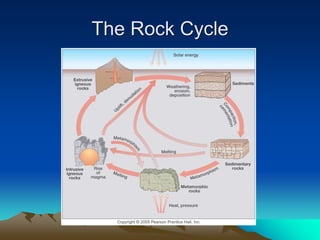
The document discusses geologic time and how rocks are dated. It describes how radiometric dating is used to determine the absolute age of rocks by measuring radioactive decay. The oldest rocks on Earth are around 3.96 billion years old. It also discusses the theory of uniformitarianism and how the same geologic processes that shape the Earth today have operated throughout its history.




































![Mount Saint Helens
• The explosion blew off 1,300 feet of the mountain's top and
sent ash and debris more than 12 miles into the sky
covering three states - Washington, Oregon, and Idaho.
Sixty two people were dead, beautiful forests and lakes
were destroyed resulting in $3 billion worth of damage.
• ‘[At about 10:00 a.m.] in the city of Yakima, Washington…a
black cloud covered the city and "snowed" ash. [Not] a
street light nor a neighbor's porch light could be seen. The
ash was so heavy it sank swimming pool covers and caved
in old roofs. Businesses and schools were closed down
and all normal activity… ceased to exist.”
From Disasters: Blowing Your Top
http://www.boisestate.edu/history/ncasner/hy210/volcano.htm](https://image.slidesharecdn.com/pglecture11-thelithosphere111416-161116071047/85/Physical-Geography-Lecture-11-The-Lithosphere-111416-43-320.jpg)