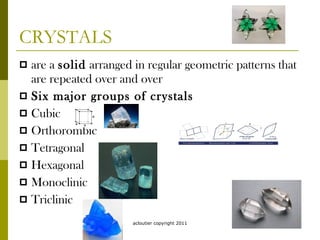
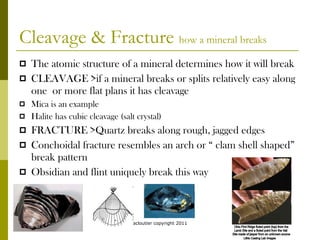
Minerals are naturally occurring inorganic solids with a specific chemical composition and crystalline structure. They form through natural geological processes in the Earth's crust and mantle. The most common minerals are silicates containing oxygen, silicon, aluminum, iron, calcium, sodium, and potassium. Minerals can form from the cooling of magma or crystallization from solution. Identification of minerals is done through analysis of physical properties like color, luster, hardness, cleavage, and other characteristics.