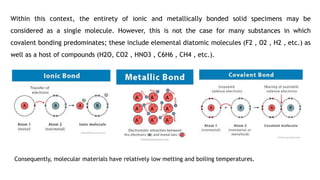
This document discusses the crystal structures of molecules and metals. It begins by defining molecules as groups of atoms bonded together, which results in relatively low melting and boiling points. Metals are considered a single molecule due to metallic bonding. There are several common crystal structures for metals including face-centered cubic, body-centered cubic, and hexagonal close-packed structures. These crystal structures are defined by the geometric arrangement of atoms in the unit cell and properties like coordination number and packing efficiency.







![Vectors and planes in a crystal lattice are described by the 3-value Miller index notation. This syntax uses the
indices ℓ, m, and n as directional parameters. Considering only (ℓ m n) planes intersecting one or more lattice
points (the lattice planes), the distance d between adjacent lattice planes is related to the (shortest) reciprocal
lattice vector orthogonal to the planes by the formula
• }} 𝑑 =
2𝜋
𝑔𝑙𝑚𝑛
For the special case of simple cubic crystals, the lattice vectors are orthogonal
and of equal length (usually denoted a); similarly for the reciprocal lattice. So,
in this common case, the Miller indices (ℓmn) and [ℓmn] both simply denote
normals/directions in Cartesian coordinates. For cubic crystals with lattice
constant a, the spacing d between adjacent (ℓmn) lattice planes is
𝑑𝑙𝑚𝑛=
𝑎
𝑙2 + 𝑚2 + 𝑛2 Miller indices](https://image.slidesharecdn.com/moleculesandmetalliccrystalstructure-210502182900/85/Molecules-and-metallic-crystal-structure-9-320.jpg)



