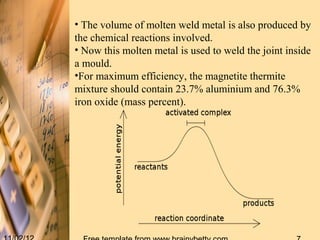
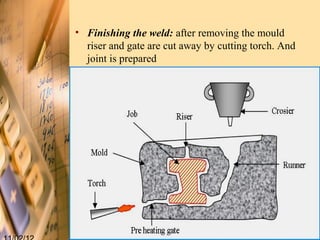
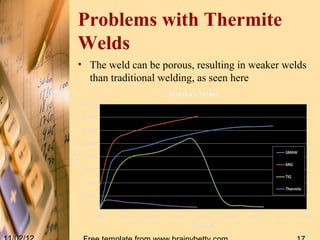
Thermit welding is a process that uses an exothermic reaction between aluminum powder and iron oxide to generate extreme heat for welding metals. It was invented in 1893 and allows for on-site welding of large metal structures like railways. The process involves mixing thermite, placing the metal pieces to be joined in a mold, igniting the thermite which reaches temperatures over 3000°C, and allowing the molten metal produced to fuse the pieces together. While it does not require external power, the welds can be porous and it is limited to ferrous metals.