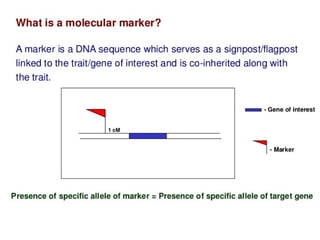
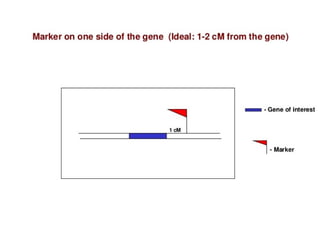
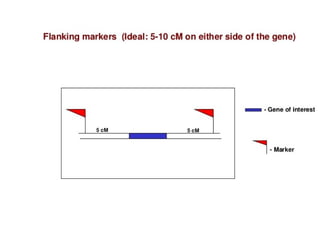
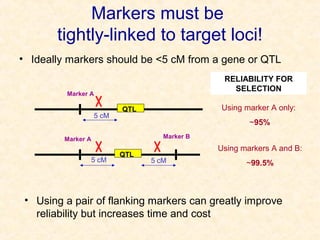
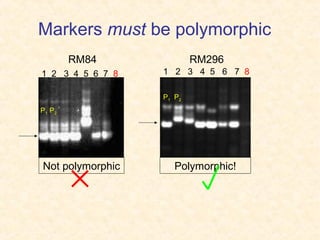
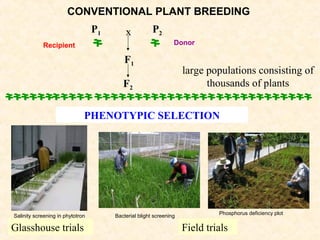
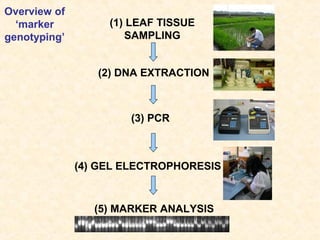
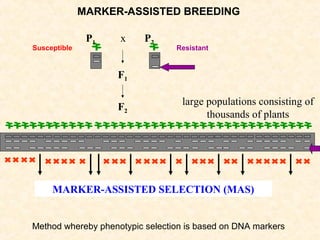
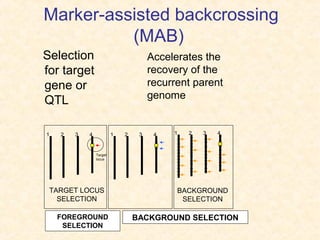
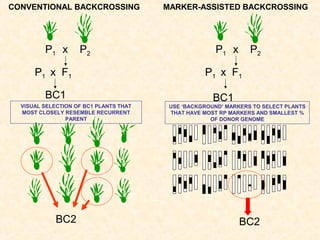
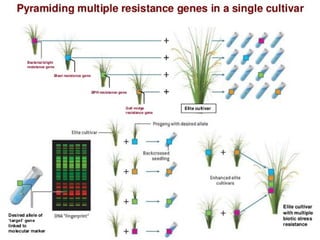
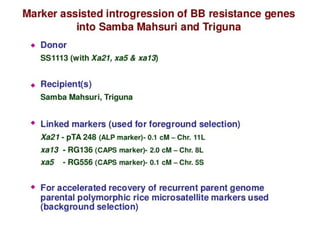
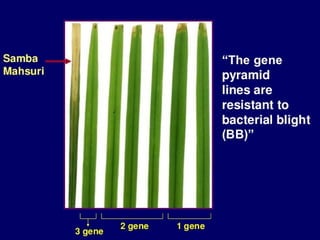
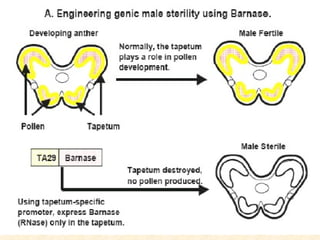
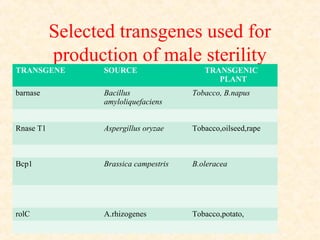
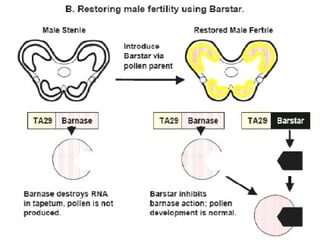
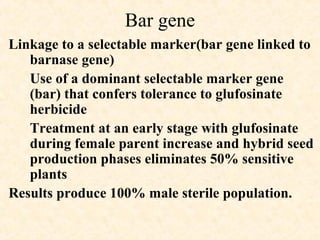
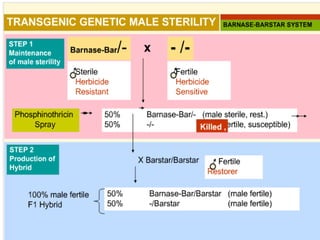
The document discusses marker-assisted selection (MAS) in plant breeding, emphasizing its advantages, current status, and challenges. MAS utilizes DNA markers to enhance the selection process for desirable traits in crops such as rice and maize, aiding in gene pyramiding and backcrossing. However, challenges such as high costs and integration of molecular genetics with traditional breeding methods remain significant barriers to its widespread adoption.