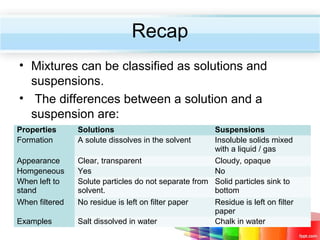
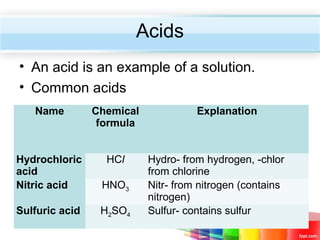
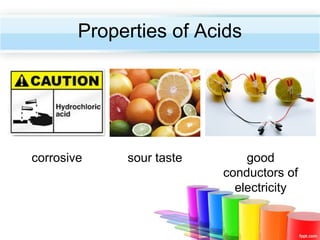
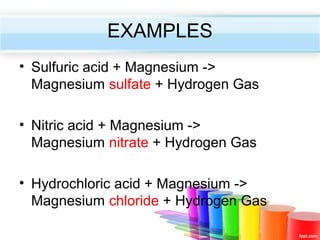
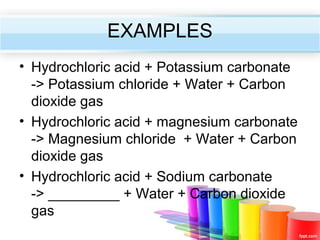
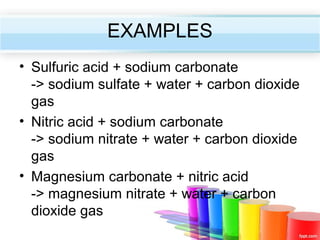
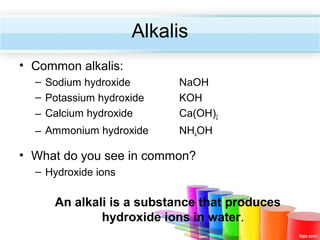
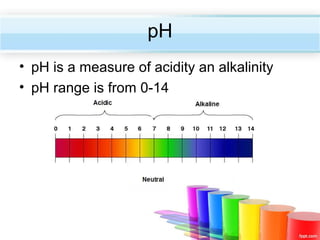
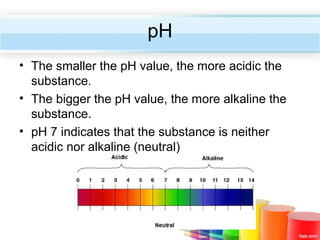
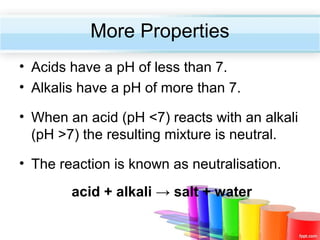
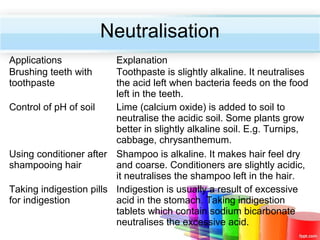
The document describes acids and alkalis. It defines acids as substances that produce hydrogen ions in water and alkalis as substances that produce hydroxide ions in water. Examples of common acids and alkalis are provided. Key chemical properties of acids and alkalis such as their reactions with metals, carbonates, and each other are outlined. The document also discusses pH, indicators, and some applications of acids and alkalis in daily life.