This experiment is investigating how temperature affects the rate of dissolving.
The temperature of the water in each beaker should also be measured and recorded.
The factors that should be kept constant are the amount of water and vitamin C tablet used. The variable factor is the temperature of the water.
Two other factors that affect the rate of dissolving are the size of particles and stirring/agitation.



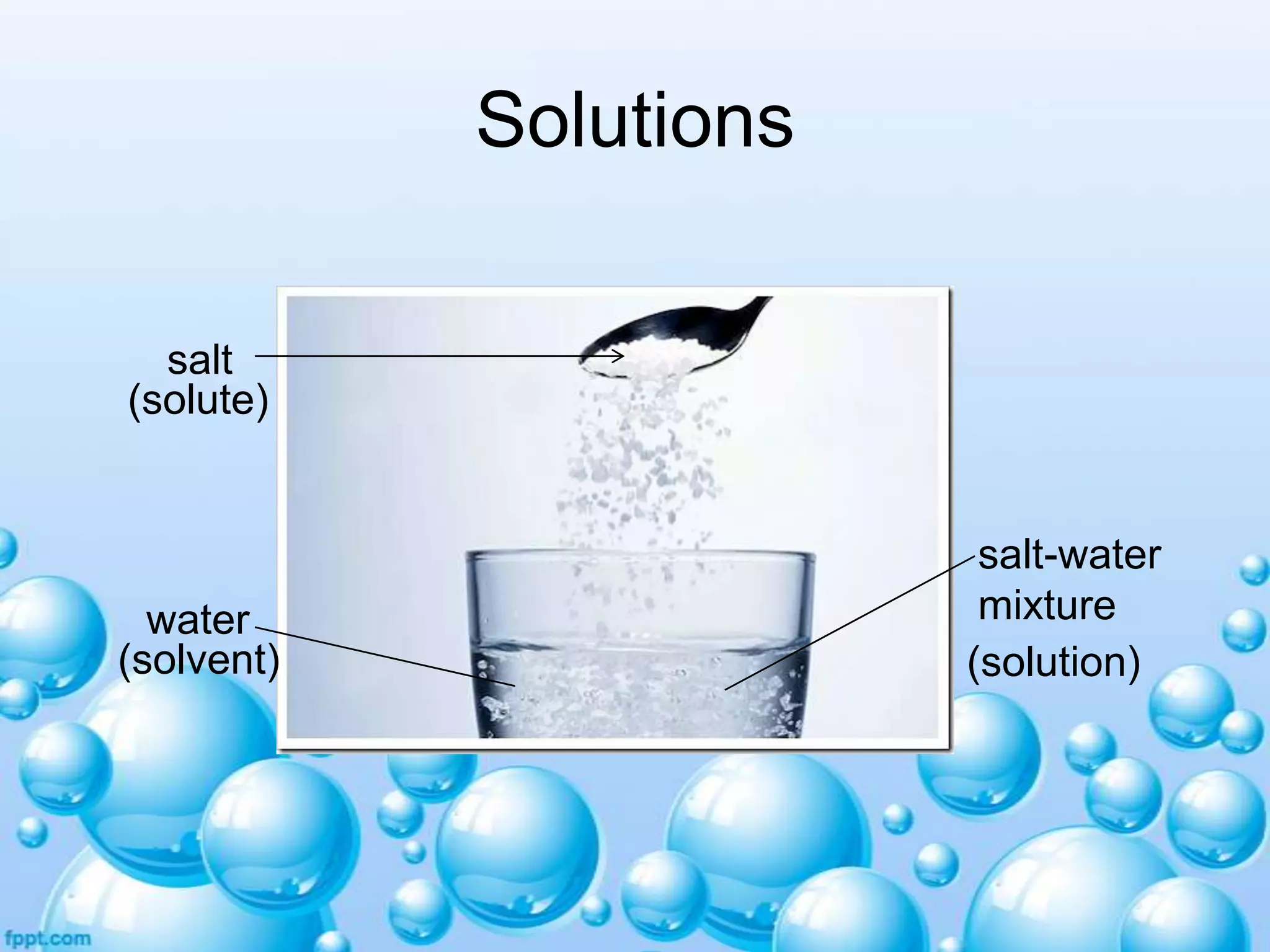


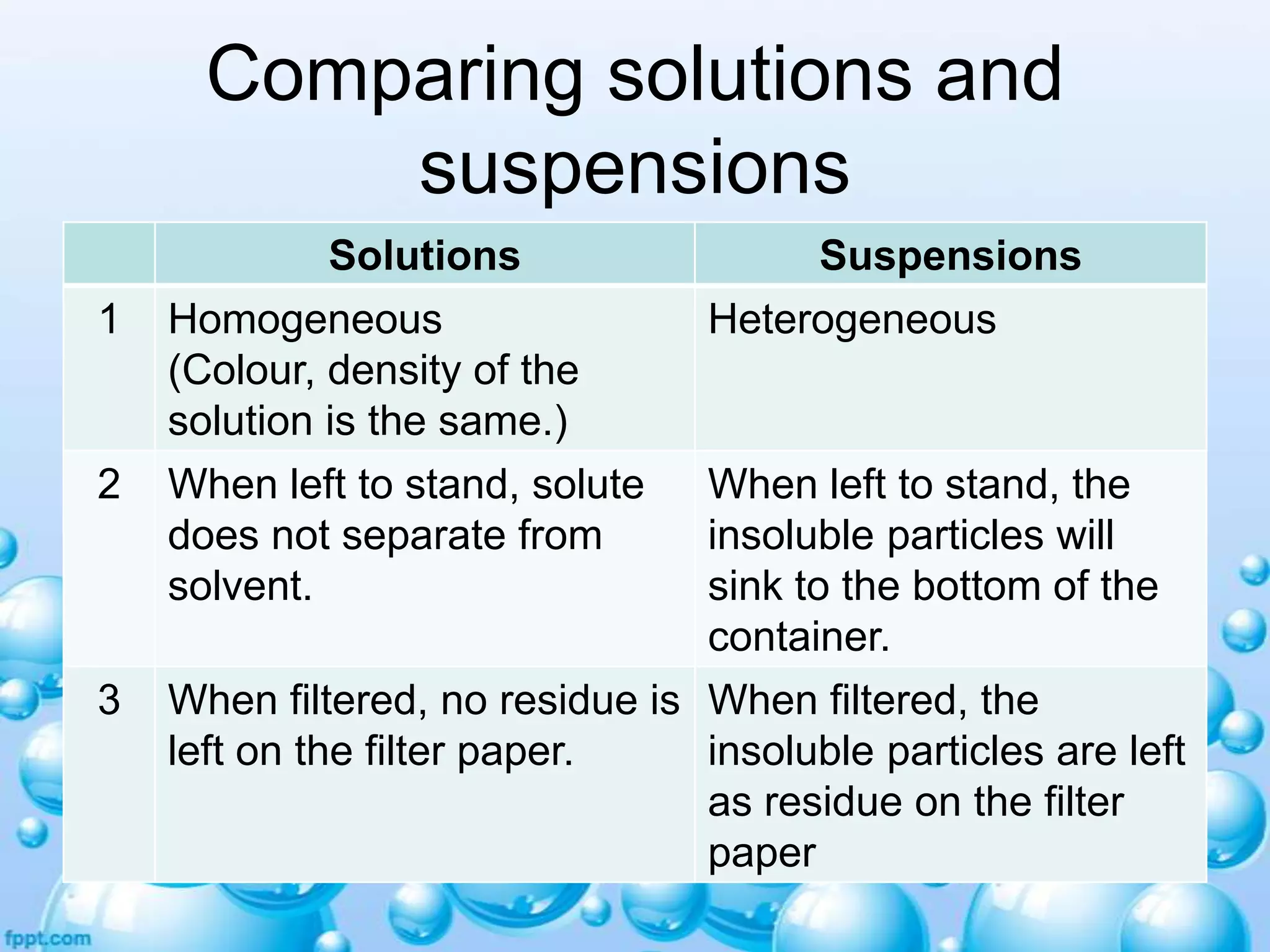






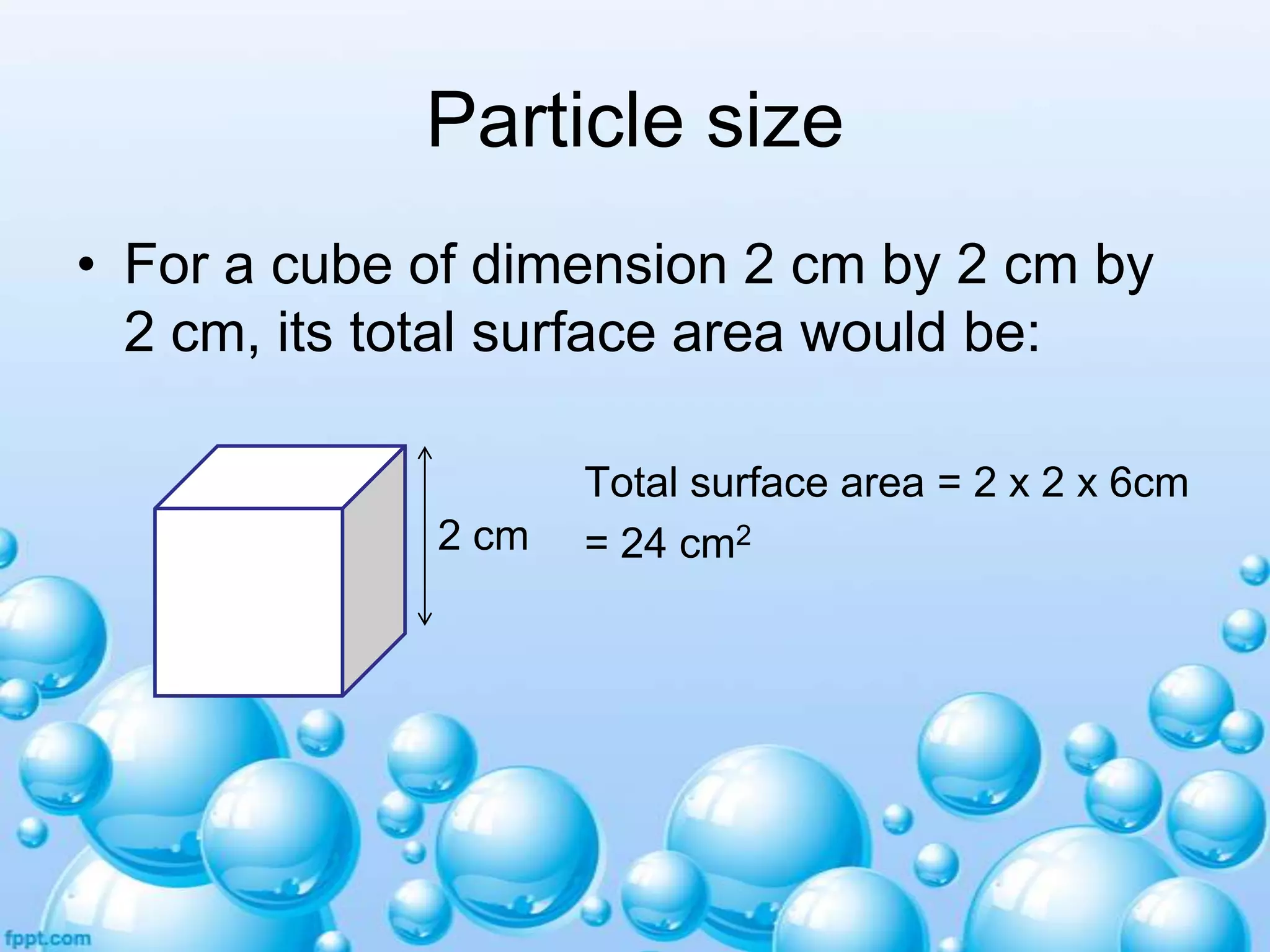
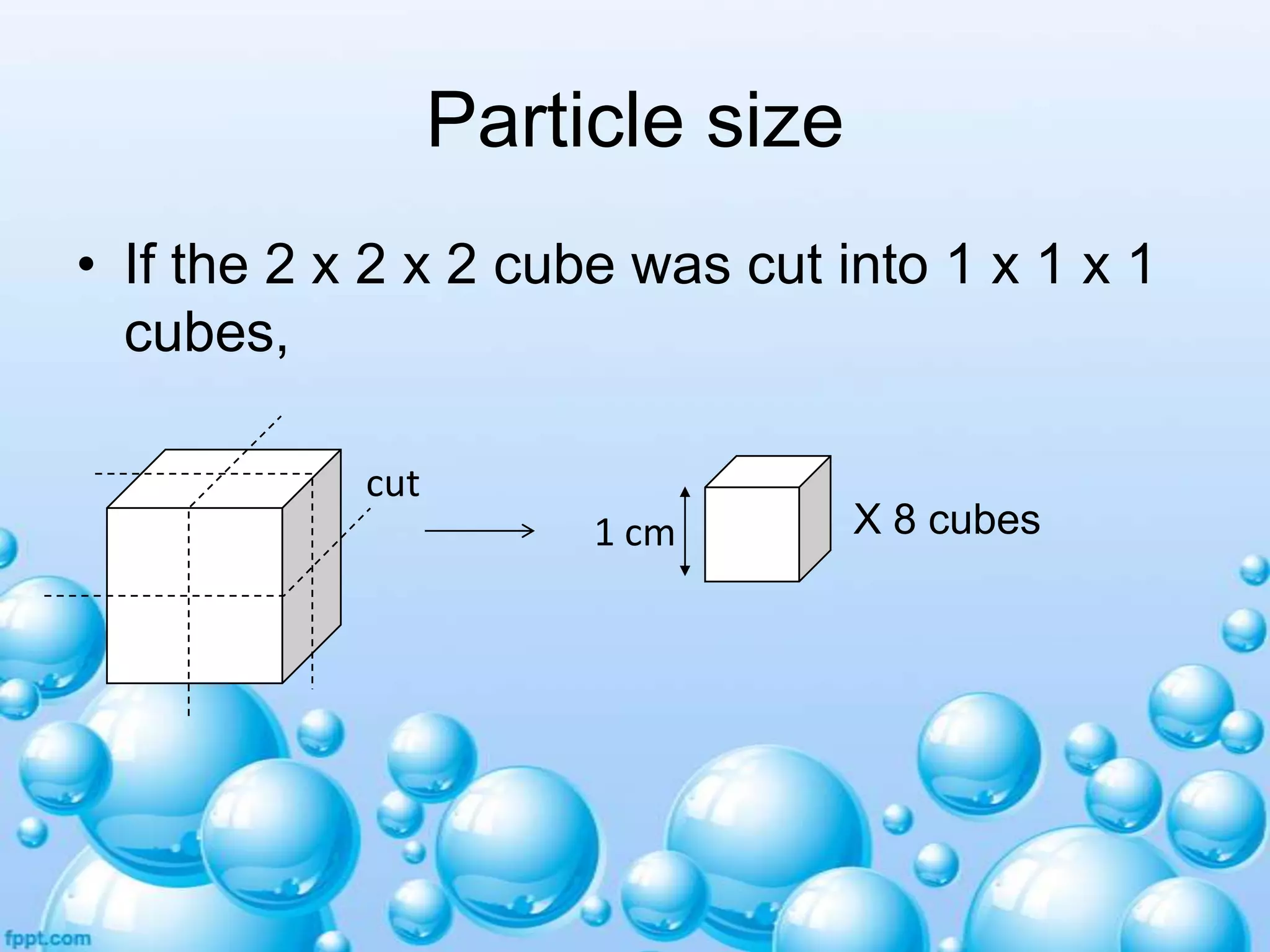
![Particle size
1 cm
X 8 cubes
Total surface area = [1 x 1 x 6] x 8
= 48 cm2
What can you conclude from this?
•When the particles are smaller,
for the same mass of substance,
there is higher total surface area
coming into contact with the
solvent, therefore smaller particles
dissolve faster.](https://image.slidesharecdn.com/solutionsandsuspensions-140101221931-phpapp01/75/Solutions-and-suspensions-20-2048.jpg)


