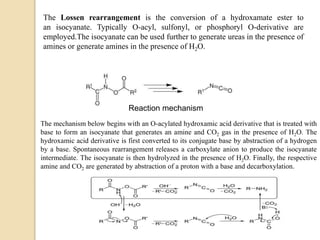
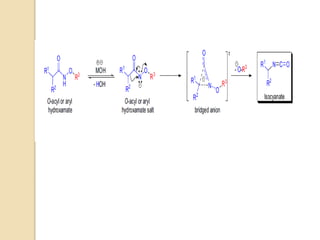
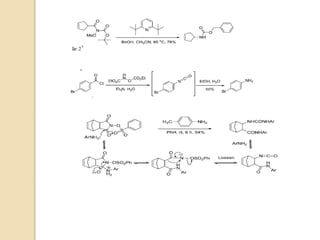
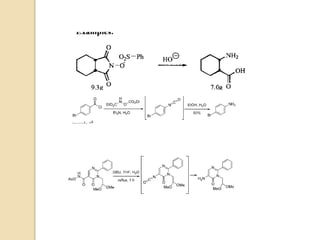
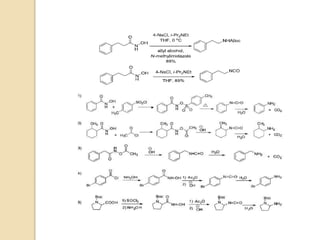
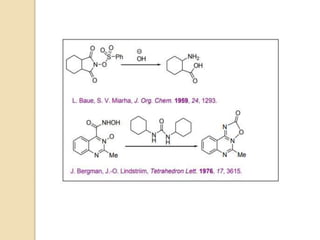
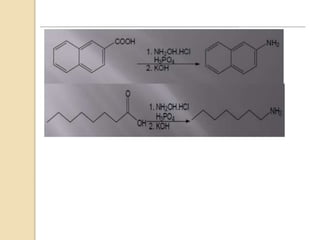
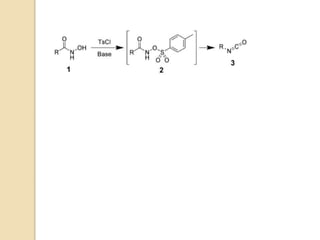
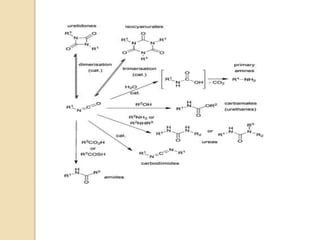
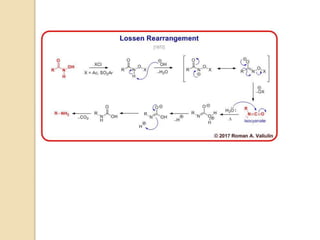
1) The Lossen rearrangement converts a hydroxamate ester to an isocyanate under basic conditions. The isocyanate can then react with water or amines.
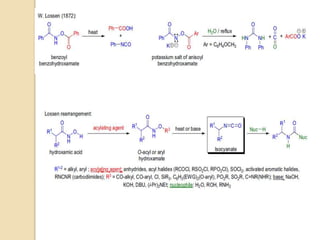
2) Wilhelm Lossen first discovered this reaction in the 1870s while studying hydroxylamines. He found that O-acylated or sulfonylated hydroxamic acids rearrange to the corresponding isocyanates upon treatment with base.
3) The reaction proceeds through an initial deprotonation to form a carboxylate anion, followed by spontaneous rearrangement to the isocyanate intermediate. This then reacts with a nucleophile like water to form an amine and carbon dioxide.