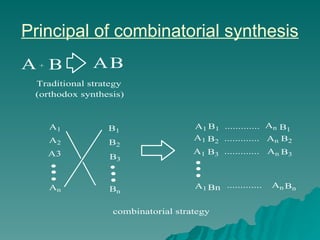
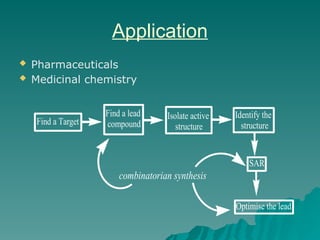
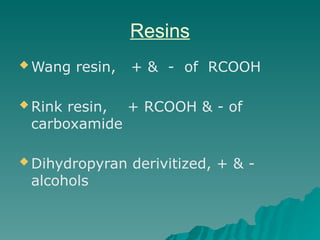
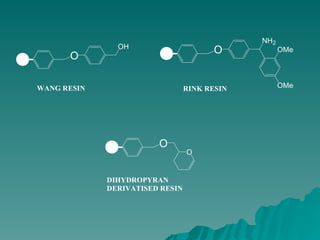
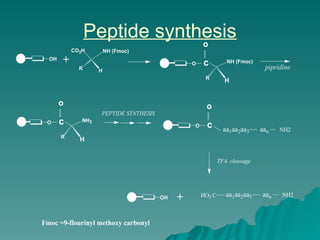
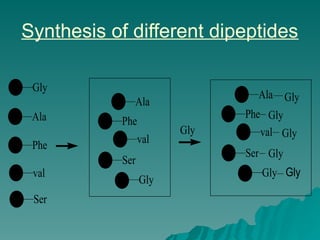
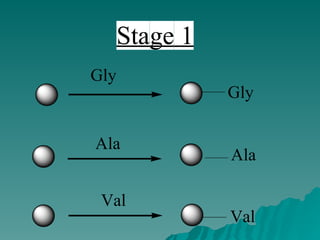
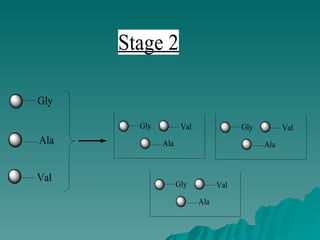
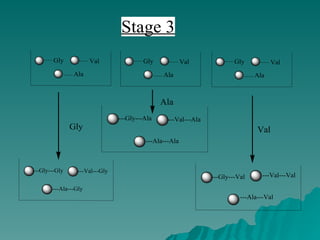
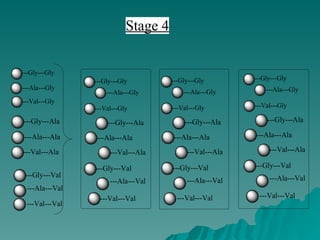
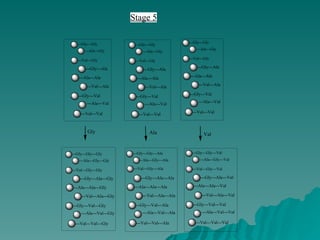
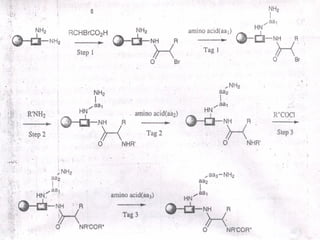
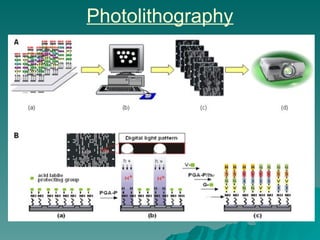
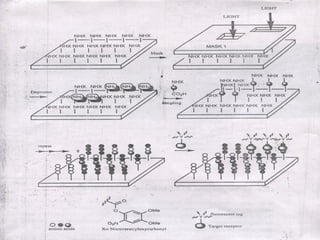
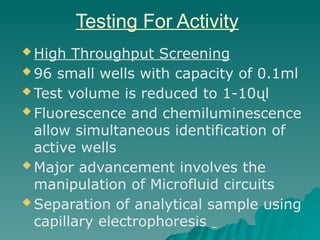
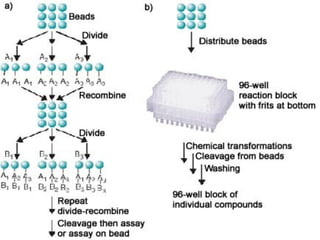
Combinatorial synthesis is a method in the pharmaceutical industry designed to rapidly produce a vast number of compounds using defined reactions and various reagents, allowing for high-throughput testing of bioactive compounds. This technique, which involves solid-phase and parallel synthesis, enhances efficiency in medicine development by reducing time and cost while increasing diversity in compounds. Additionally, methods such as deconvolution, tagging, and photolithography are employed to isolate active compounds and synthesize complex molecules, including peptides and inhibitors, effectively advancing research in medicinal chemistry.