1. The document discusses drug discovery and development, outlining the need to address unmet medical needs like new diseases as well as the costs of existing therapies.
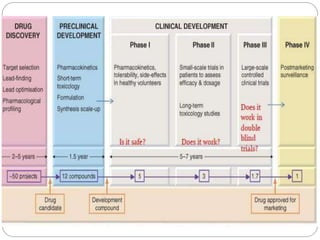
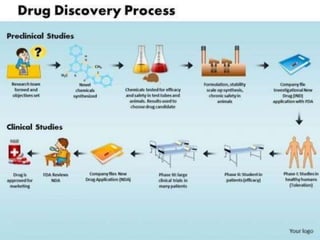
2. It describes the historical aspects of clinical trials and regulations dating back to the 1500s, and outlines the modern drug development process including discovery, preclinical studies, and clinical trials through the various phases.
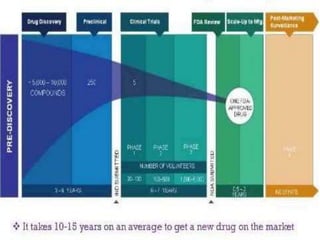
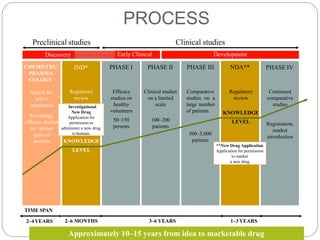
3. The drug development pathway involves discovery, preclinical development including chemistry/pharmacology and toxicology studies on animals, and clinical development including Phase I-III trials on volunteers and patients, with the goal of regulatory approval and market introduction over approximately 10-15 years.