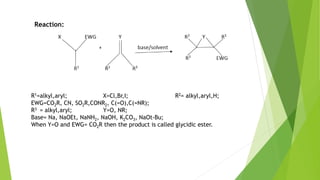
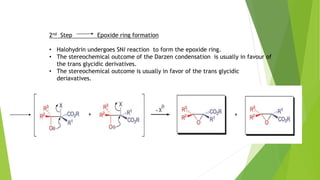
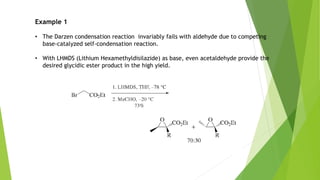
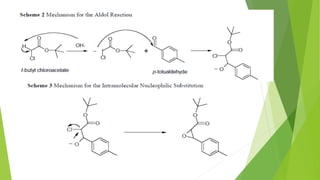
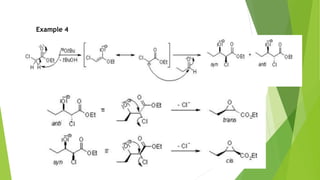
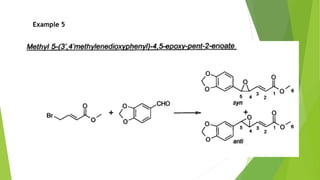
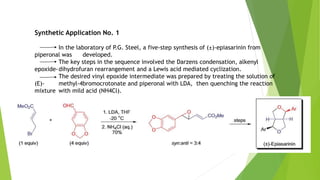
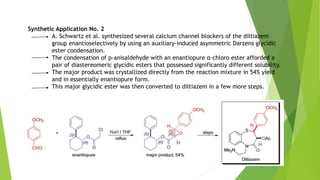
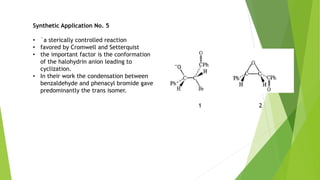
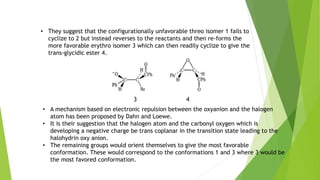
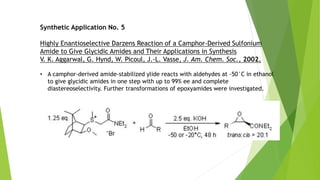
The document summarizes the Darzen condensation reaction, which involves the formation of α,β-epoxy esters (glycidic esters) from aldehydes/ketones and α-halo esters under basic conditions. It provides background on the reaction's history and development. The reaction mechanism proceeds through an initial aldol reaction followed by epoxide ring formation. Examples and applications in multi-step syntheses are described. Limitations include the reaction failing for some aldehydes due to self-condensation and difficultly obtaining high, sole product yields needed for kinetic studies.