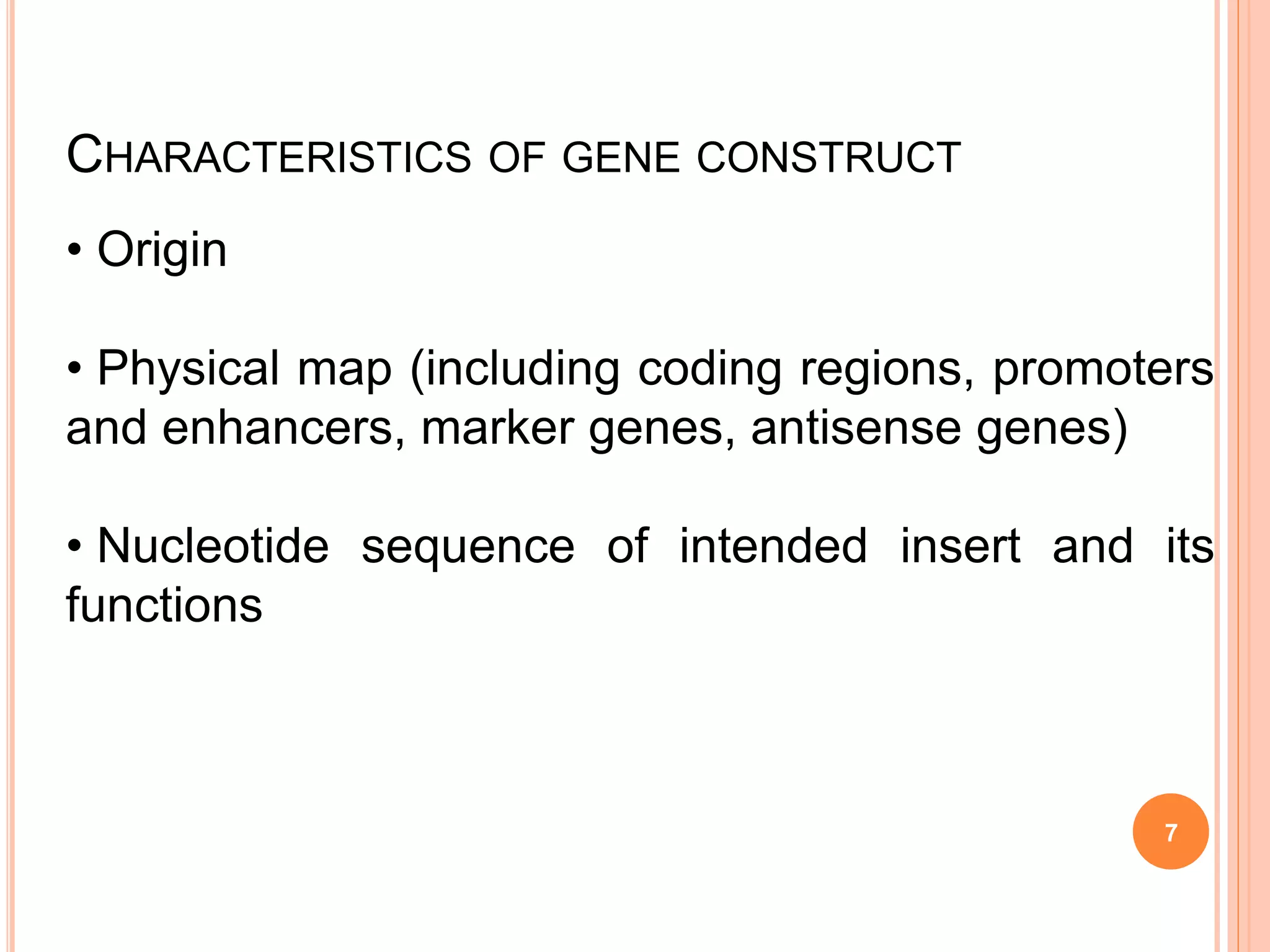
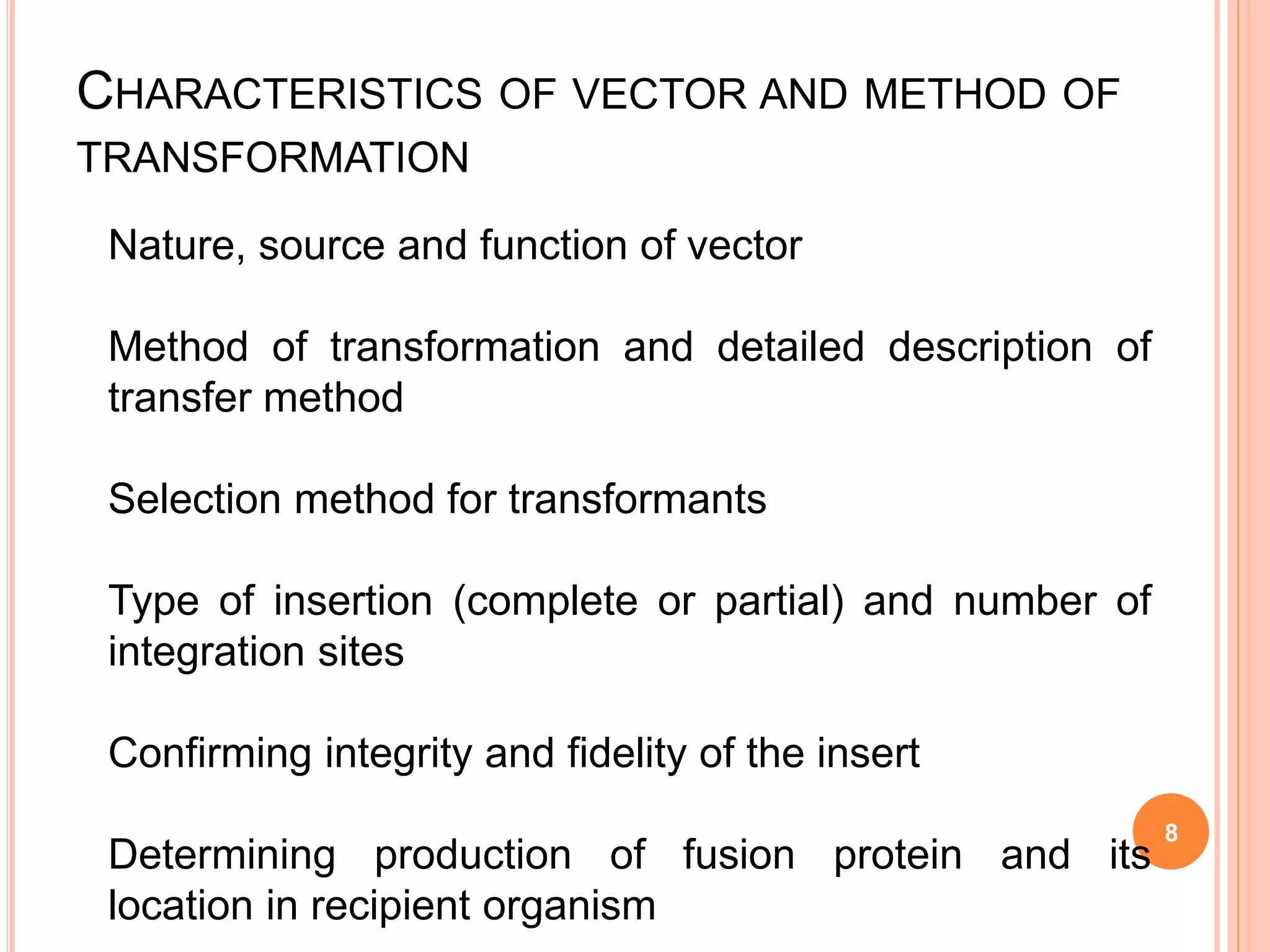
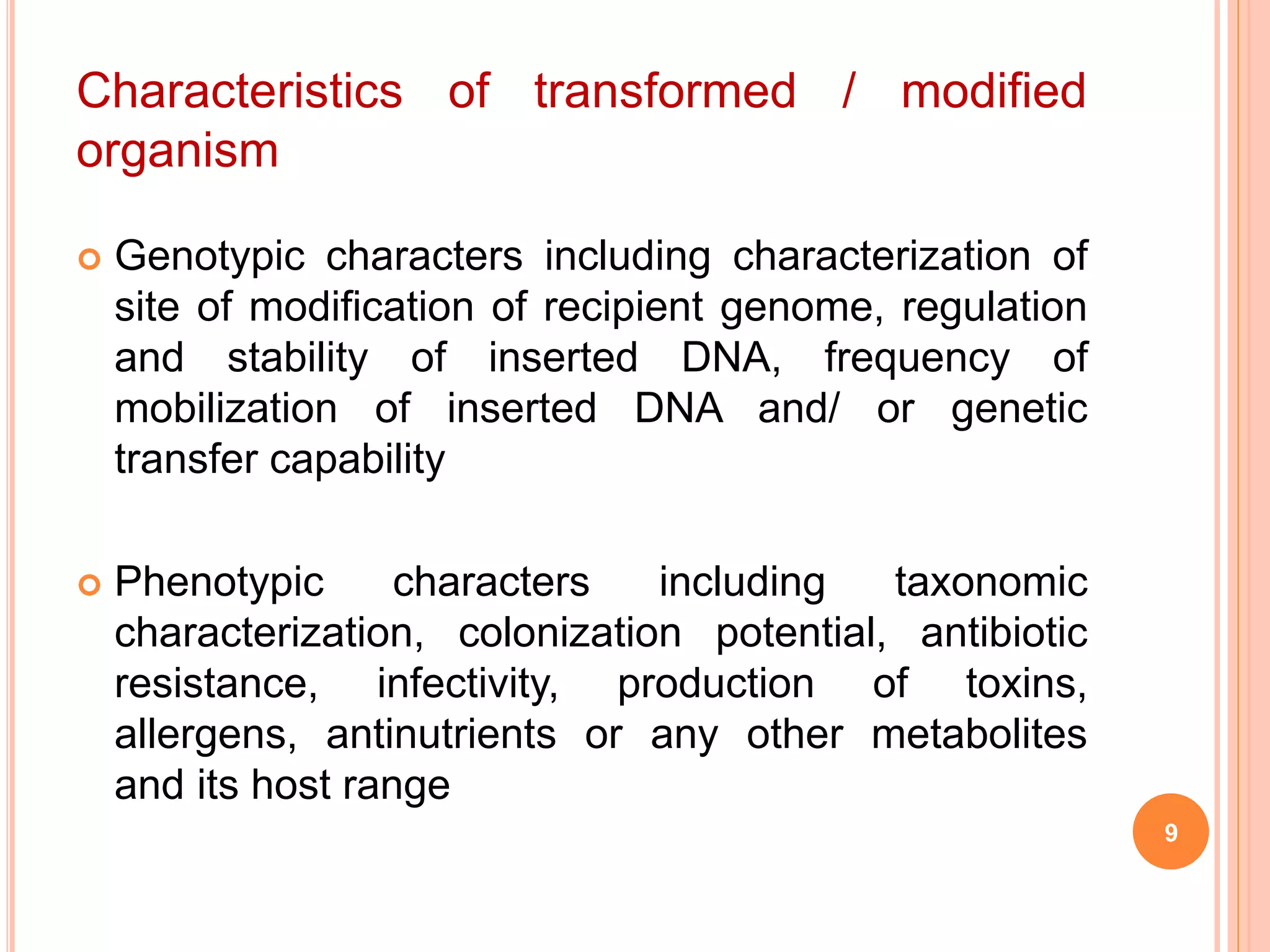
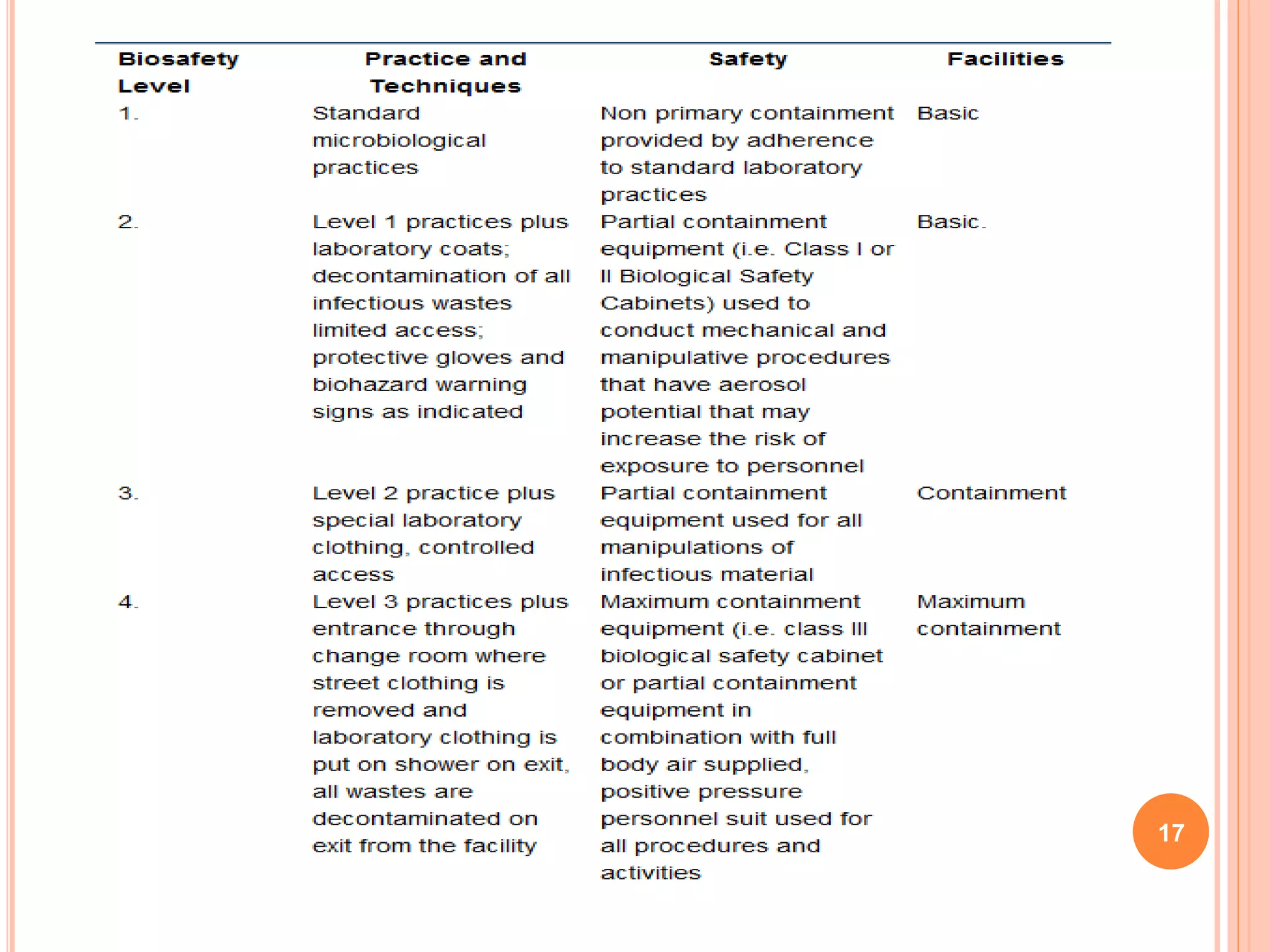
This document discusses risk assessment in genetic engineering. It outlines characteristics to consider for the donor organism, host/recipient organism, gene construct, vector, and transformed organism. These include taxonomy, genetics, pathogenicity, expression of gene products, and potential health and environmental impacts. Experiments are categorized based on risk from I to III, with category I needing only notification and category III requiring review and approval. General safety considerations are outlined related to containment facilities, equipment, and disposal of materials. The document provides guidance for conducting thorough risk assessments of genetic engineering experiments.