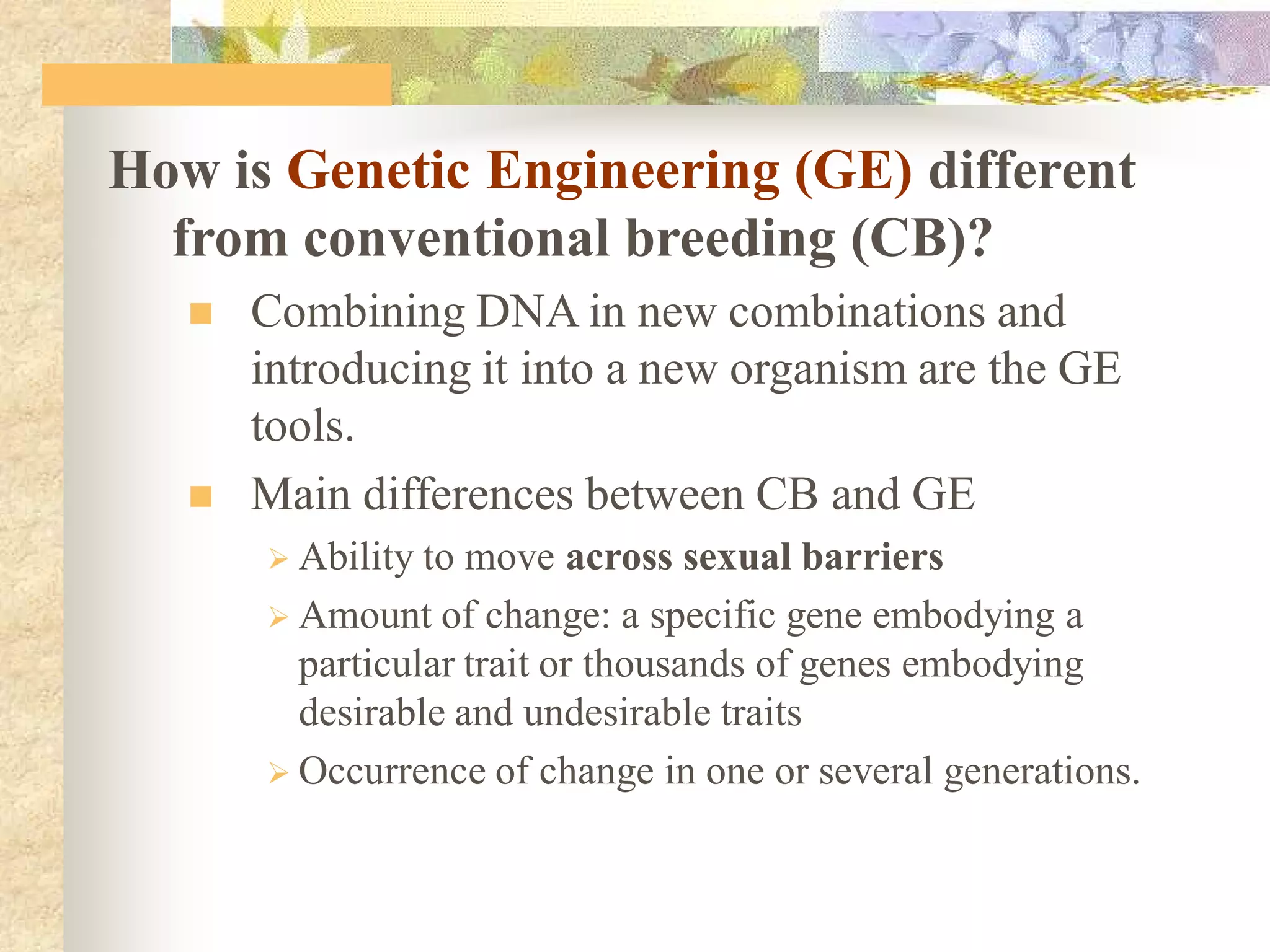
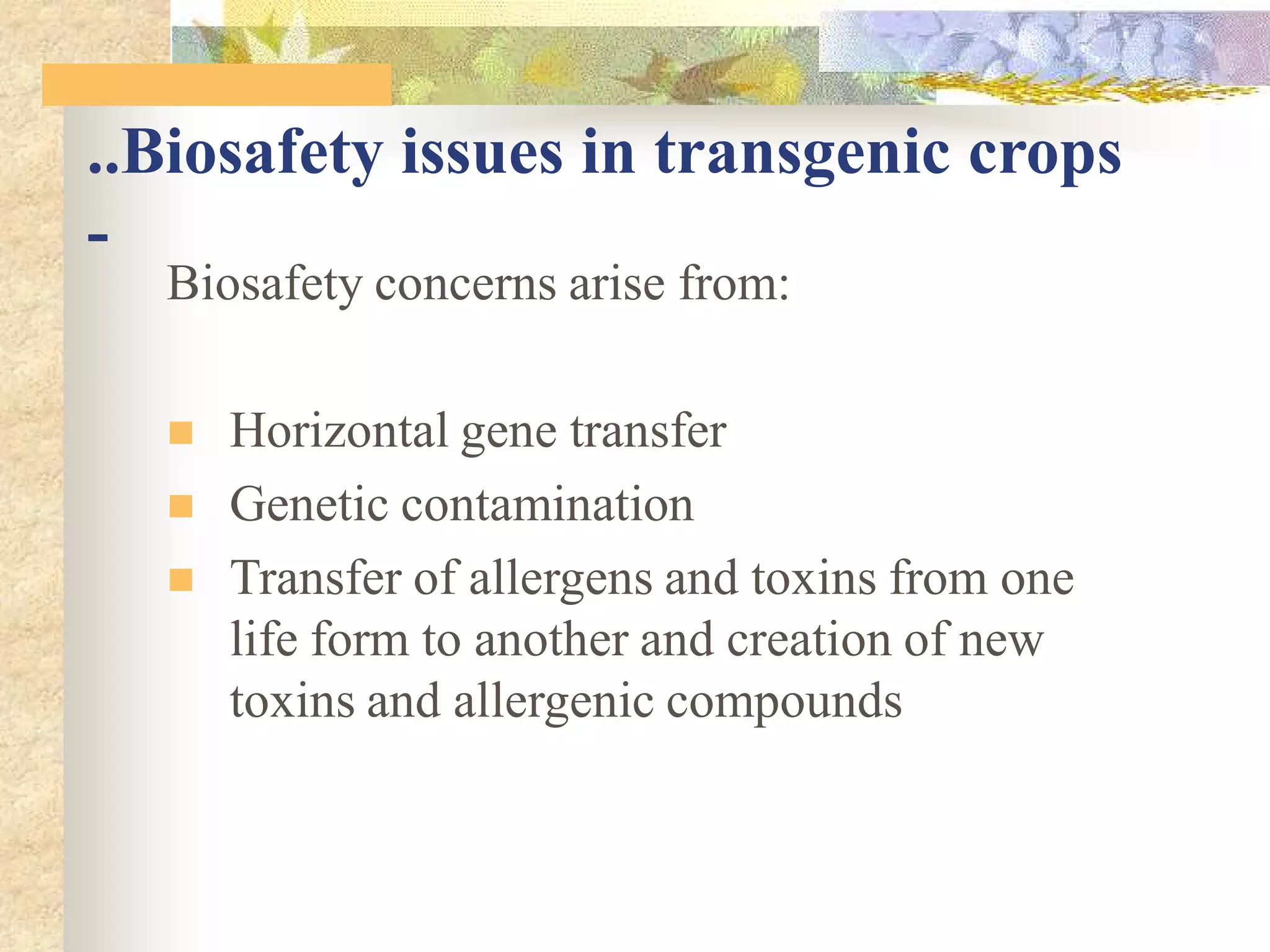
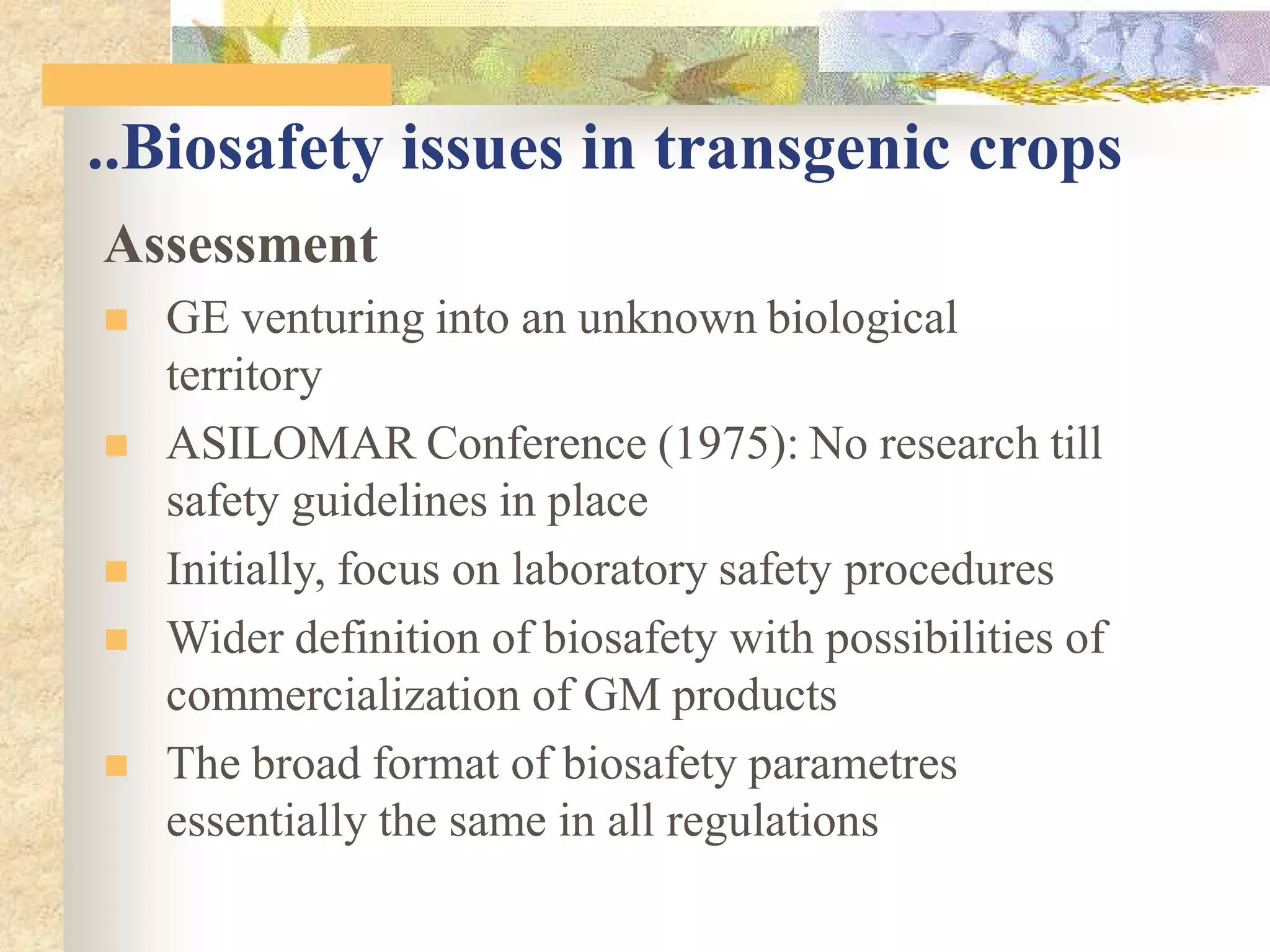
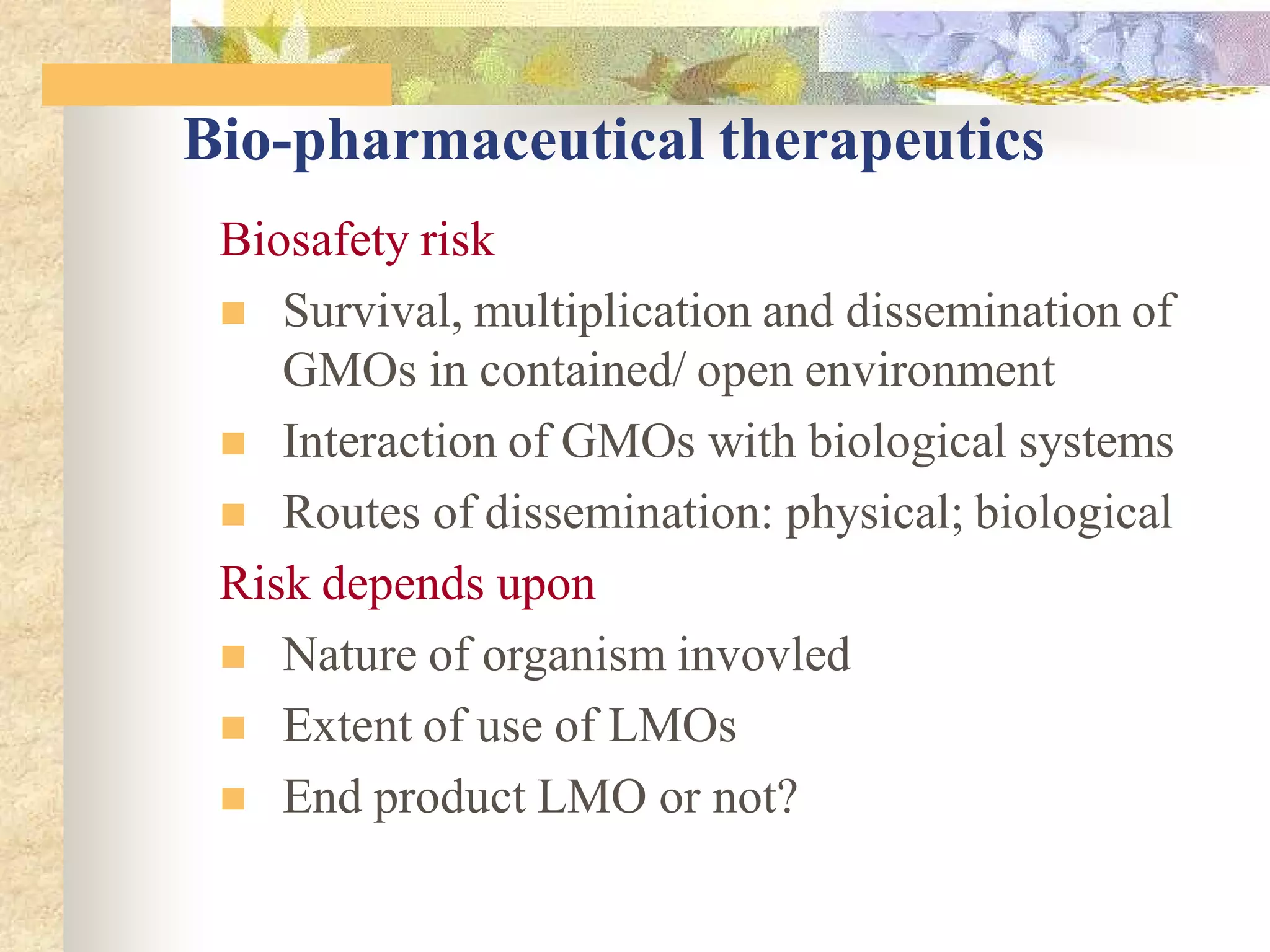
This document discusses biosafety issues related to modern biotechnology. It provides background on the emergence of environmentalism and key international agreements related to biosafety, such as the Convention on Biodiversity and the Cartagena Protocol on Biosafety. The document then discusses differences between genetic engineering and conventional breeding. It examines debates around whether genetic engineering is inherently unsafe and notes that case-by-case scientific risk assessments are needed. The document outlines biosafety concerns for transgenic crops and bio-pharmaceuticals. It emphasizes that rigorous scientific assessment and risk mitigation are important to ensure biosafety.


![ Environmentalism emerged as a distinct
development in the last forty years.
Emergence of “pressure groups” in the sixties
First Earth Day (1970)
The United Nations Conference on the Human
Environment and Development (1972)
The Brundtland Report: our Common Future
(1987)
The Rio Earth Summit (1992)
Convention on Biodiversity (CBD) [1992]
Cartagena Protocol on Biosafety (CPB) [1993]
International Evolution](https://image.slidesharecdn.com/05environmentalandbiosafetyissues-180520164632/75/05-environmental-and-biosafety-issues-3-2048.jpg)
![Convention of Biodiversity (CBD) [1992]
Focus: conservation and sustainable use of
biodiversity
Recognized the potential of modern biotechnology
for human well being
Took cognizance that modern biotechnology
could have serious effects on environment and
health
Article 8(g) emphasized the need to regulate the
risks associated with the use of LMOS.
Article 19(3) set the stage for a legally binding
international instrument about biosafety.](https://image.slidesharecdn.com/05environmentalandbiosafetyissues-180520164632/75/05-environmental-and-biosafety-issues-4-2048.jpg)