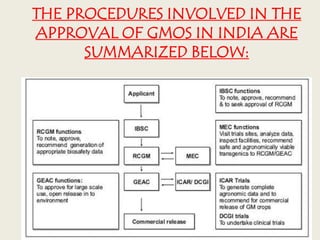
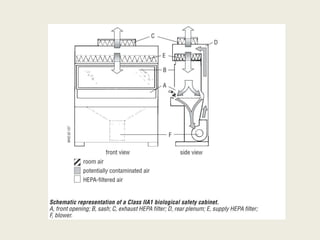
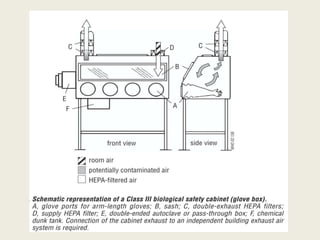
The document discusses biosafety definitions, biological risk assessment, and guidelines for working with genetically modified organisms (GMOs). It defines biosafety as ensuring safety in using, handling, and disposing of biological organisms. Risk assessment of GMOs involves characterizing the agent, identifying hazards, evaluating risks, and applying management strategies. The guidelines classify GMOs based on their history of safe use and specify containment levels and approvals required for field testing.