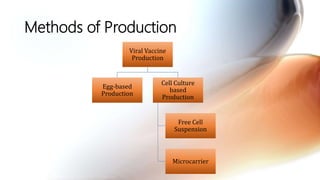
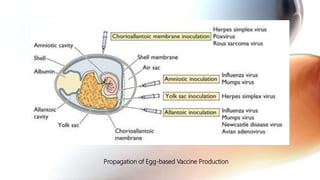
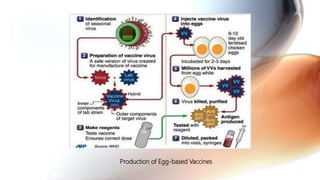
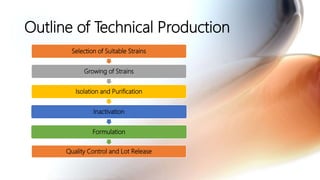
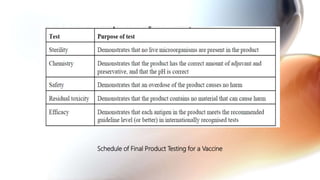
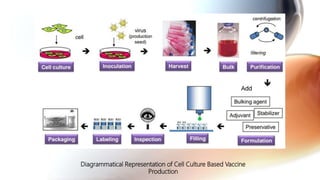
This document discusses cell culture based vaccine production. It begins by introducing different types of vaccines and traditional egg-based vaccine production methods and their limitations. It then describes the importance and advantages of cell culture based methods, including types of cells used. The key steps of the cell culture based production process are outlined, including strain selection, bulk production, purification, virus inactivation, formulation, quality control testing, and lot release. Specific cell culture based vaccines for influenza, rabies, dengue, and Ebola are discussed. The conclusion emphasizes the potential for cell culture to replace egg-based methods by producing vaccines faster and in larger quantities to meet global demand.