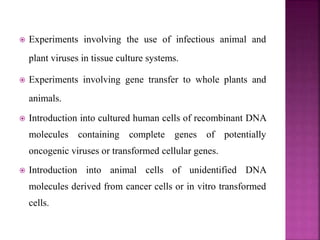
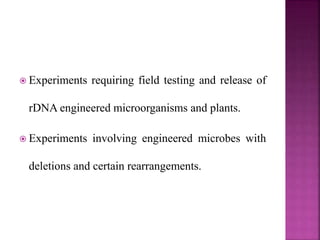
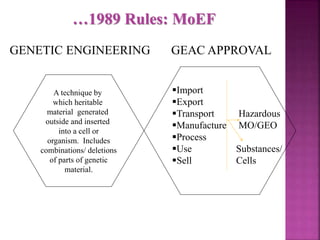
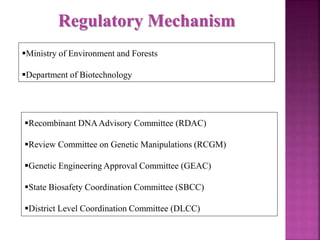
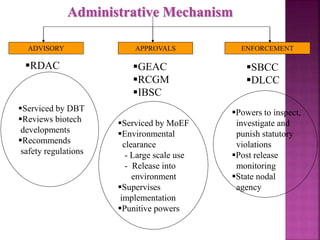
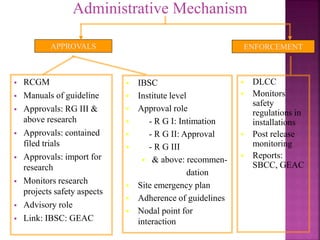
The document outlines India's biosafety guidelines for research and development activities involving genetically modified organisms (GMOs). It discusses the four biosafety levels established by the Department of Biotechnology (DBT) and the containment facilities recommended for each level. It also categorizes experiments into three categories - Category I experiments which are exempt from approval, Category II experiments which require institutional biosafety committee intimation, and Category III experiments which require prior approval from competent authorities. The document provides examples of experiments that fall under each category and discusses the roles of different committees like IBSC, RCGM, GEAC that oversee and regulate GMO research in India.