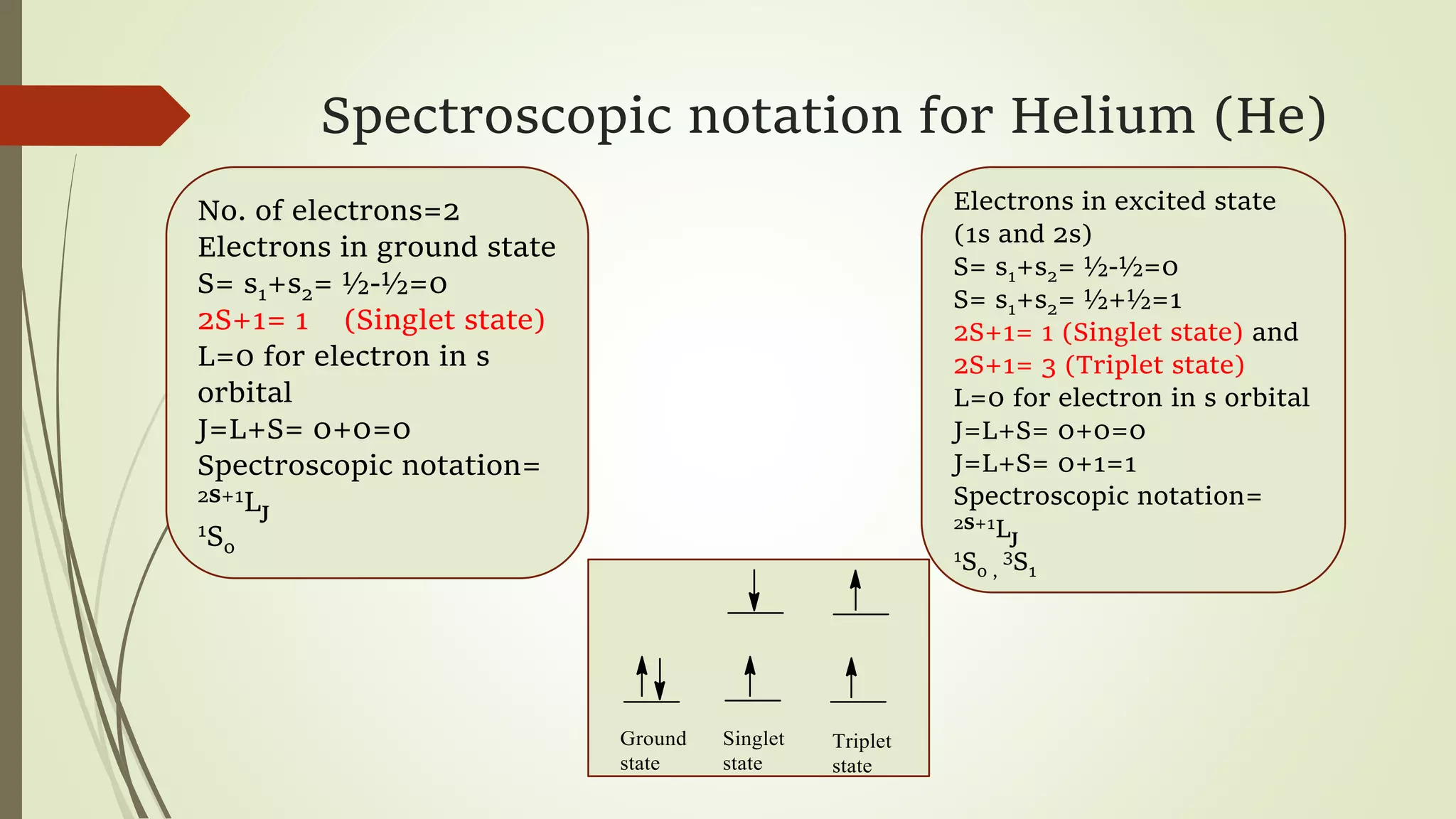
The document discusses spectroscopic notations used to describe the quantum states of atoms and ions. It introduces the principal, azimuthal, magnetic, and spin quantum numbers that are used to quantitatively describe observed atomic transitions. The spectroscopic notation describes the atomic state using these quantum numbers, written as 2S+1LJ, where S, L, and J are the spin, orbital, and total angular momentum quantum numbers. Examples are given for the ground and excited states of helium.