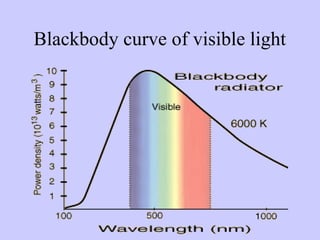
This document discusses spectroscopy and how it is used to analyze light from stars and determine their composition. It provides three key points:
1) A spectroscope separates white light into a spectrum of wavelengths that is unique for each chemical element. The pattern of wavelengths emitted is like a fingerprint that identifies the element.
2) When elements are heated, they emit light across the visible spectrum in distinct patterns called emission spectra. Observing these spectra in starlight allows scientists to determine which elements are present on distant stars and galaxies.
3) All light from stars and galaxies is redshifted due to the expansion of the universe, providing evidence that the universe has been expanding since the Big Bang over 14 billion years ago.