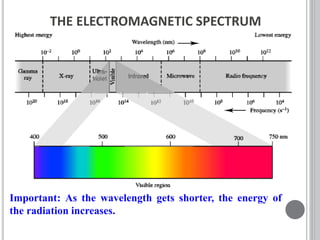
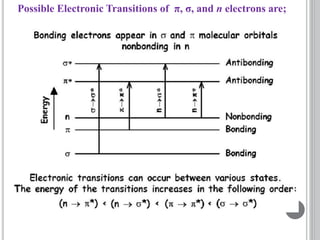
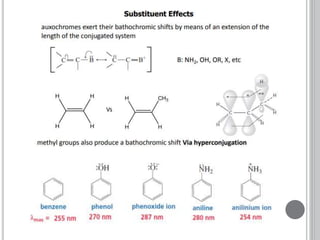
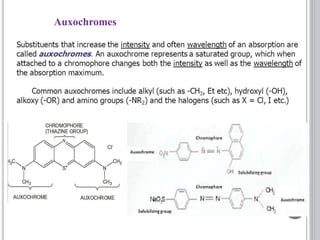
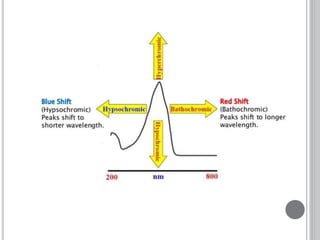
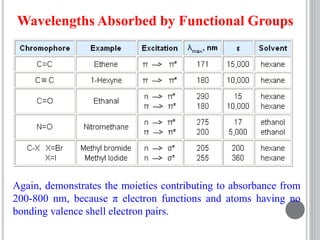
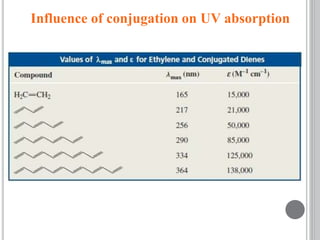
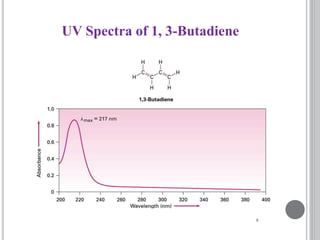
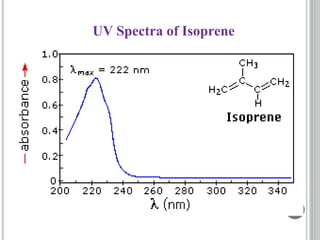
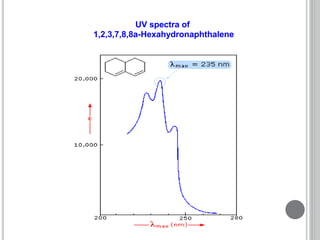
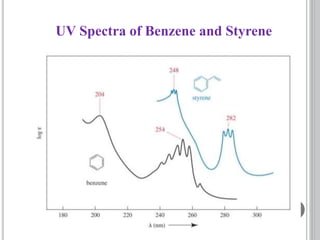
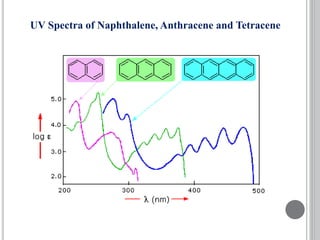
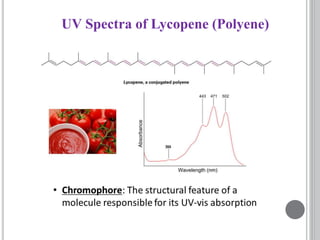
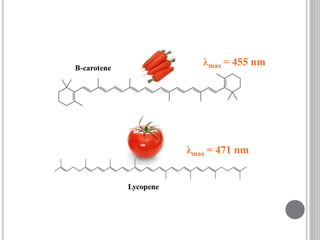
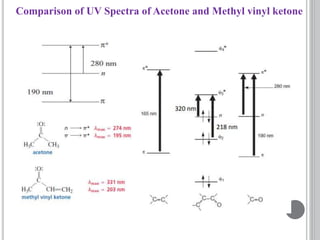
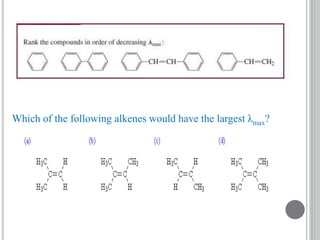
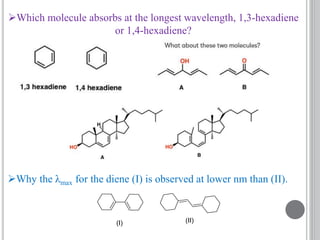
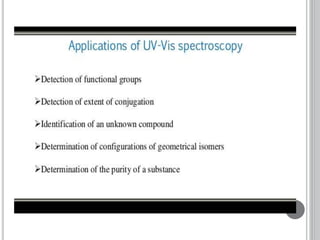
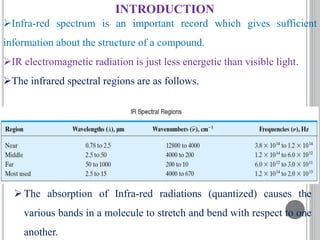
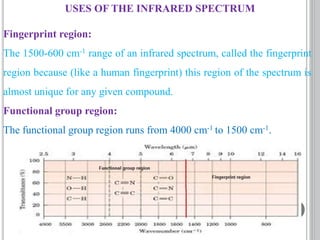
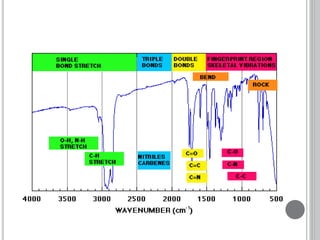
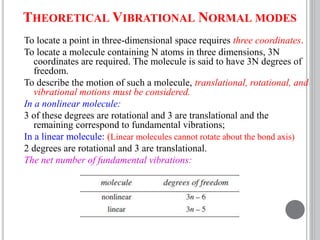
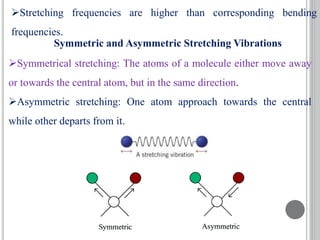
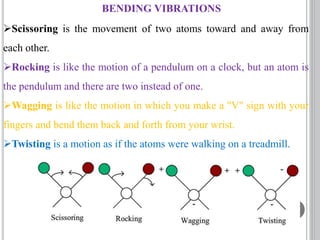
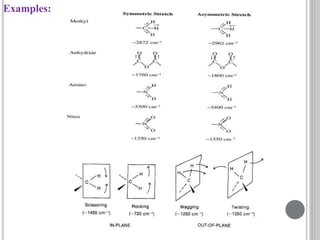
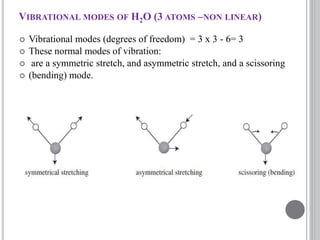
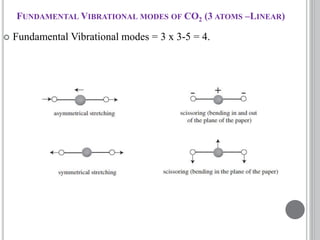
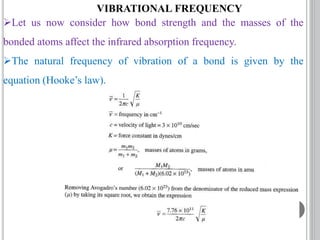
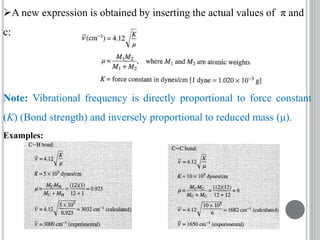
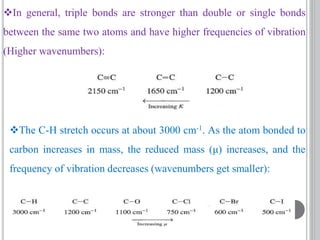
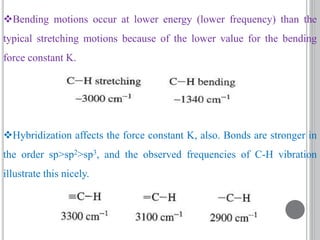
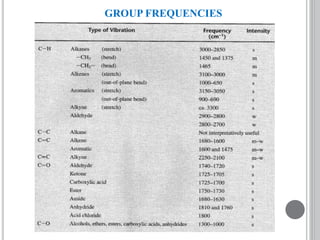
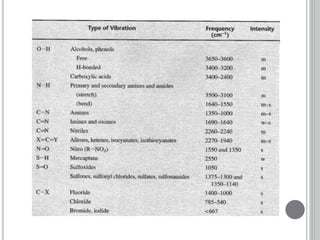
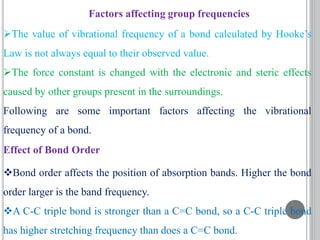
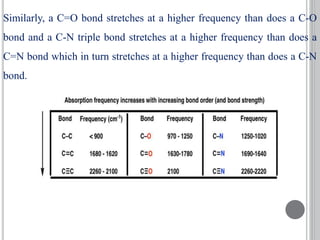
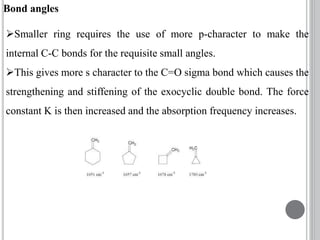
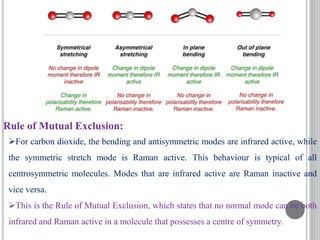
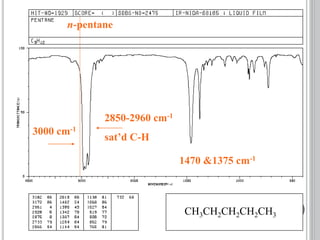
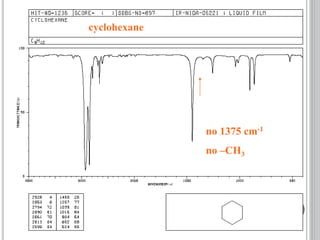
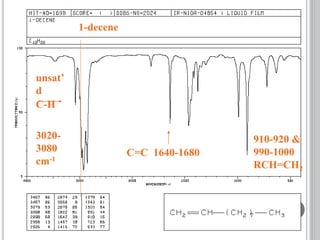
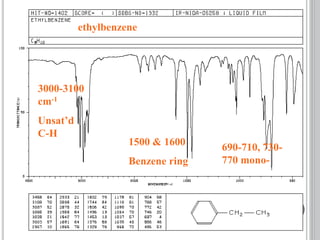
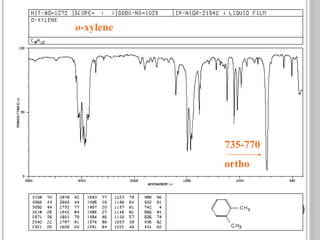
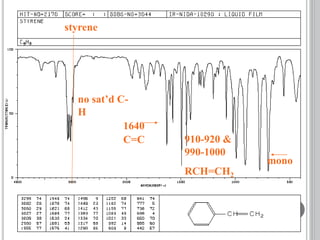
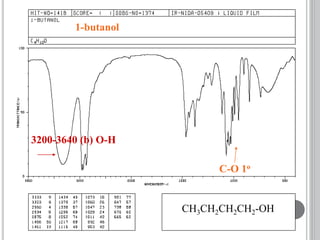
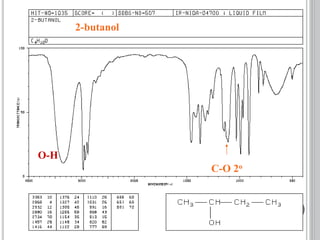
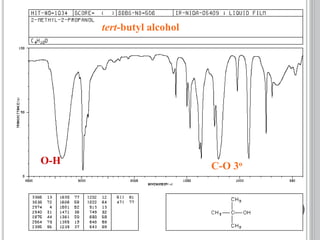
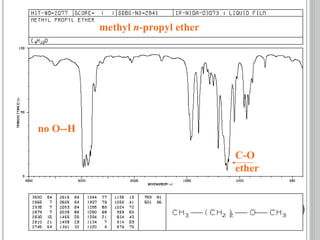
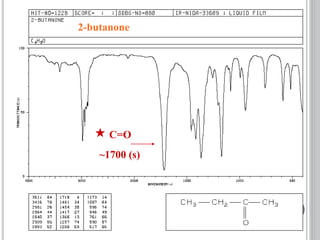
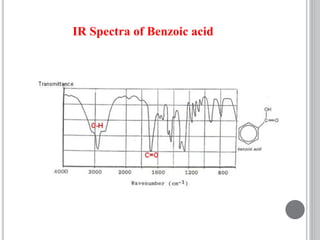
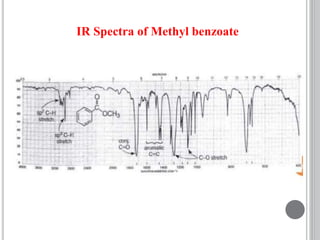
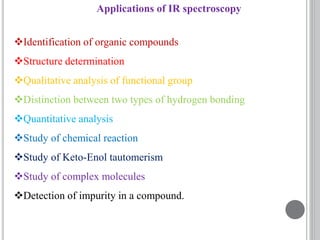
This document provides an overview of UV-visible and infrared spectroscopy, detailing how electromagnetic radiation interacts with matter and the principles underlying absorption techniques. It discusses the energy levels affected by ultraviolet and visible wavelengths, and the vibrations and rotations induced by infrared radiation, along with the relevance of electronic transitions and molecular structure in spectral analysis. The document also highlights key concepts, terminology, and applications of spectroscopy in the identification and analysis of various compounds.