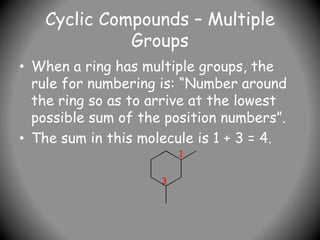
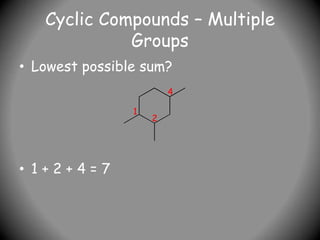
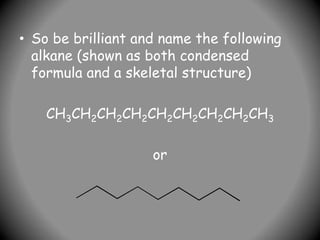
1) IUPAC nomenclature provides systematic names for organic compounds based on their structure. The name consists of prefixes to indicate substituents, a parent chain or ring, and suffixes to indicate the compound class.
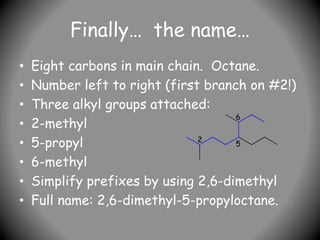
2) Alkyl groups that are substituents are named as prefixes according to the number of carbons (methyl, ethyl, propyl, etc.) and their position of attachment.
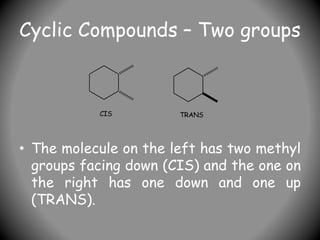
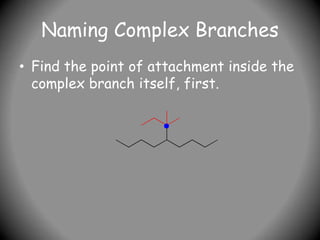
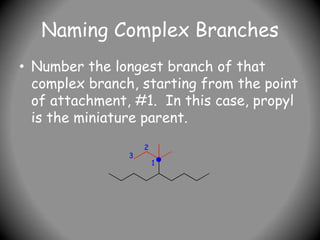
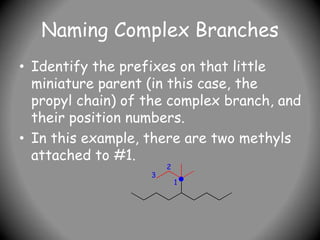
3) For cyclic compounds, the largest ring or chain is the parent. Smaller rings or chains become prefixes like "cyclobutyl".






















![Sec-Butyl Groups
• The other possibility is to form the
fragment one of the central carbons:
CH3CHCH2CH3, which is called “sec-
butyl”. [Note that the carbon second
from the left only has three bonds – so
that’s where its bonding to the main
chain]](https://image.slidesharecdn.com/nomenclature-221026142541-5780f371/85/no-men-30-320.jpg)










![Naming Complex Branches
• Write the full name, placing the
complex branch inside brackets. This
complex branch is attached to #5 of
the original main chain so:
• 5-[1,1-dimethylpropyl]nonane is the
name.
1
2
3](https://image.slidesharecdn.com/nomenclature-221026142541-5780f371/85/no-men-51-320.jpg)