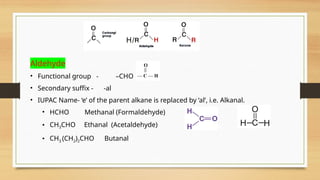
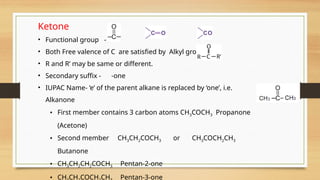
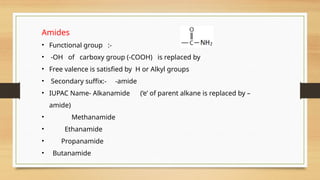
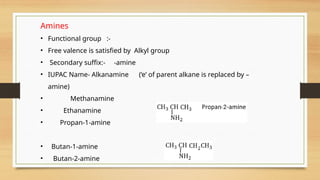
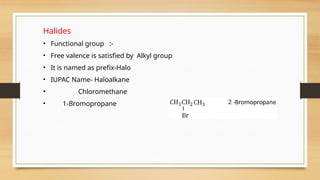
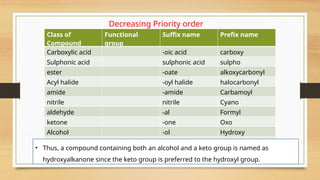
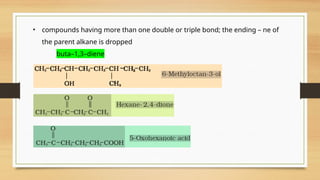
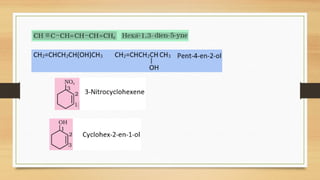
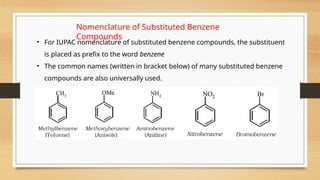
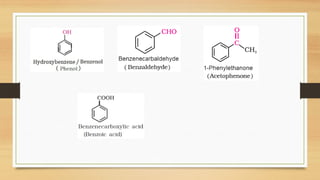
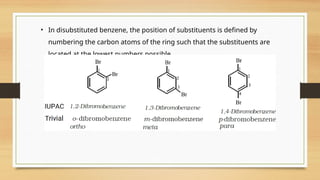
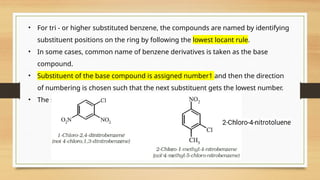
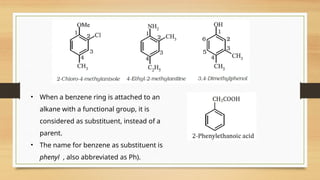
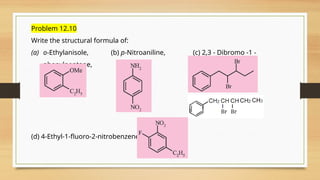
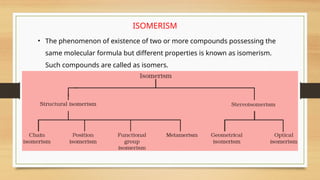
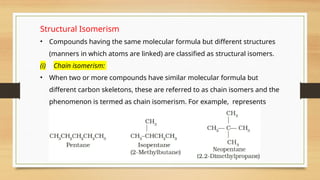
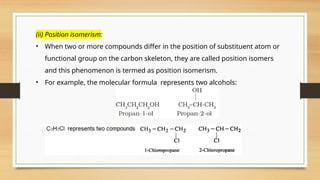
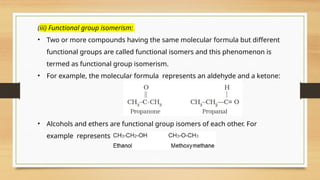
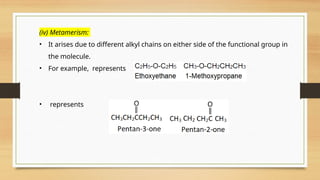
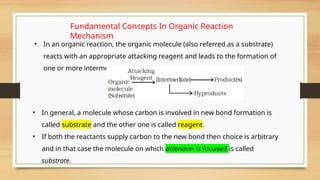
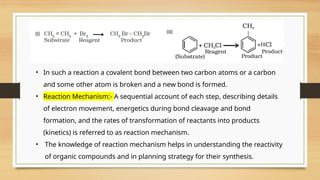
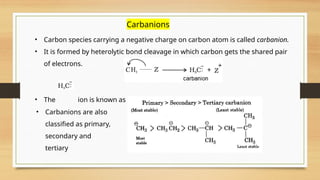
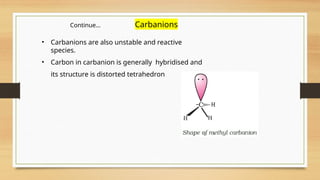
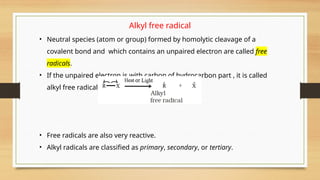
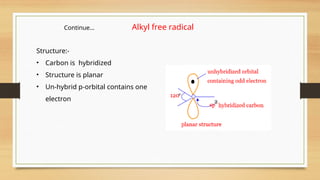
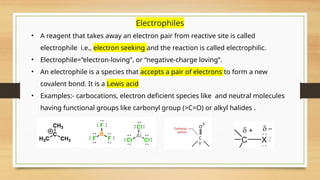
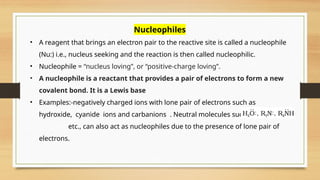
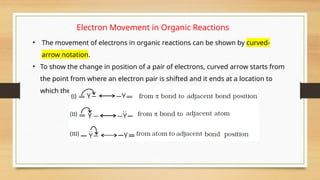
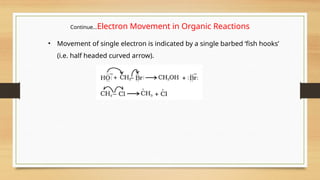
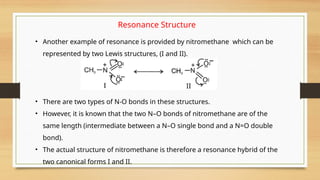
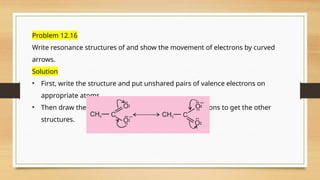
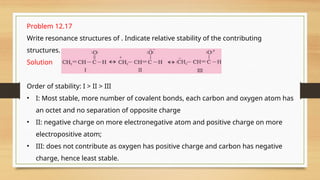
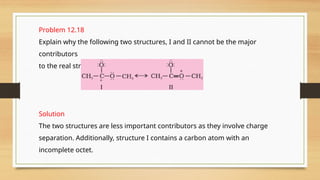
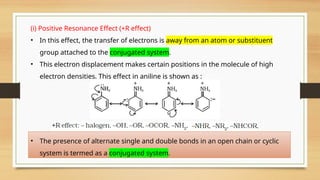
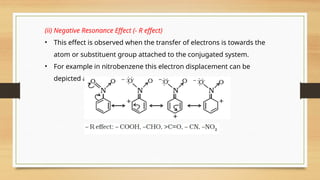
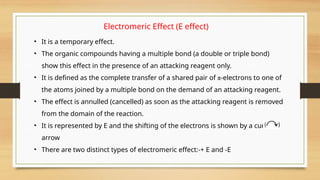
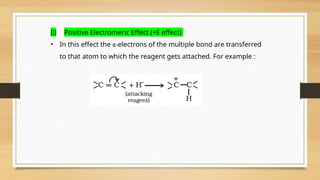
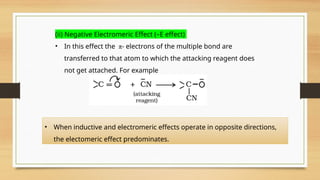
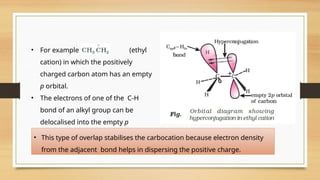
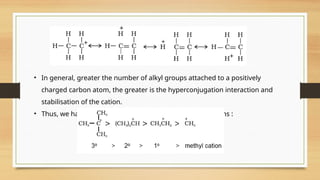
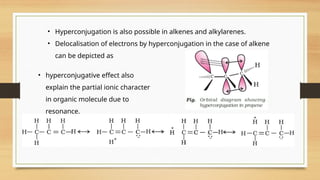
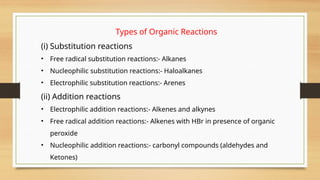
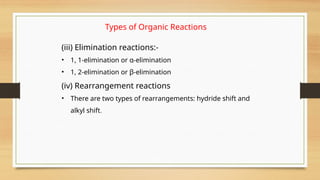
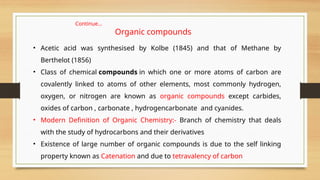
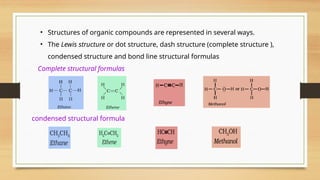
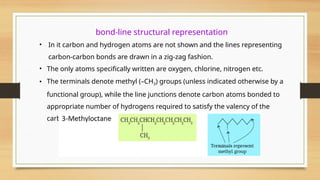
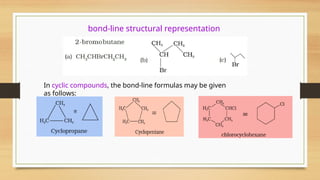
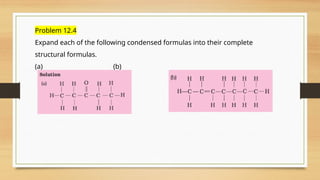
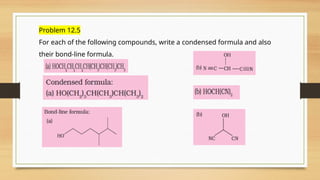
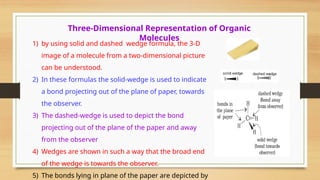
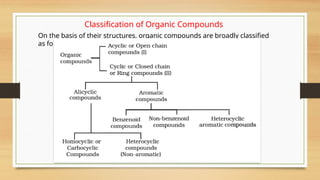
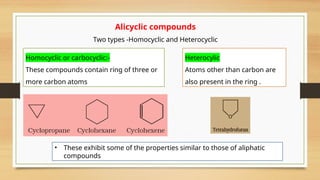
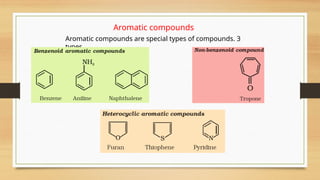
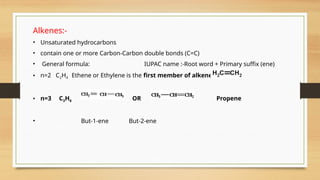
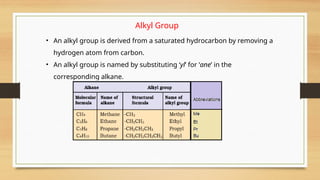
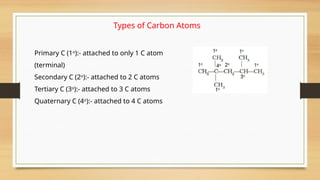
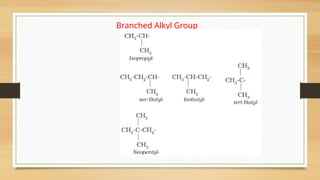
The document outlines the basic principles of organic chemistry, including the shapes of carbon compounds, hybridization, and the vital force theory's rejection. It covers structural representations, classifications of organic compounds, and IUPAC nomenclature for naming straight-chain and branched alkanes, alkenes, alkynes, functional groups, and polyfunctional compounds. Key concepts such as the self-linking property of carbon, hybridization's effects on bond characteristics, and systematic naming conventions are also discussed.













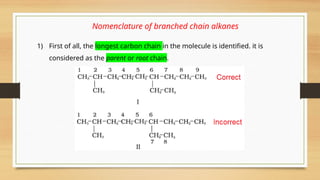



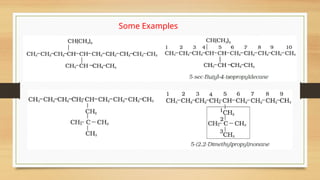
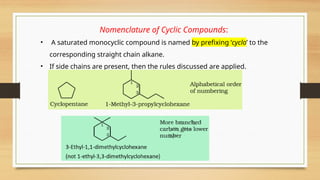
![• If there is more then one chain of equal size, then that chain is to be selected
which contains more number of side chains.
• After selection of the chain, numbering is to be done from the end closer to the
substituent.
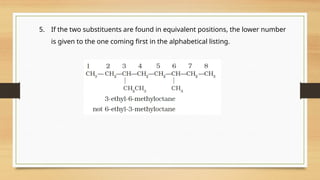
5-(2-Ethylbutyl)-3,3-dimethyldecane
[and not 5-(2,2-Dimethylbutyl)-3-ethyldecane]](https://image.slidesharecdn.com/organicchemistry-somebasicprinciplesandtechniques-250109143246-dc307d6e/85/Organic-Chemistry-Some-Basic-Principles-and-Techniques-pptx-38-320.jpg)