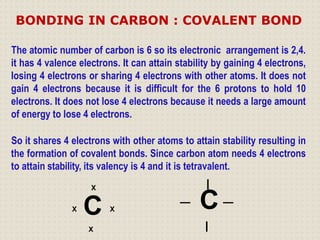
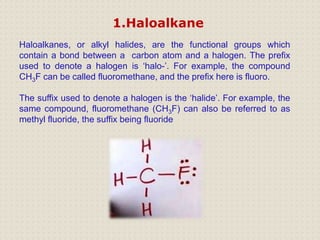
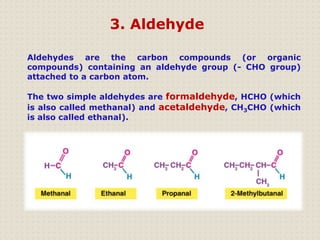
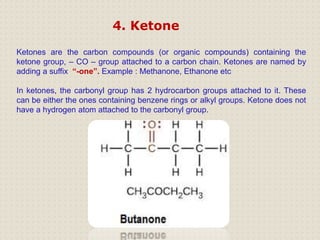
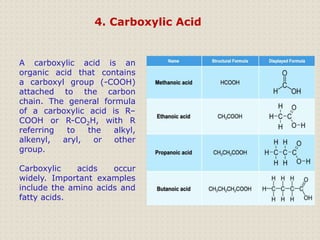
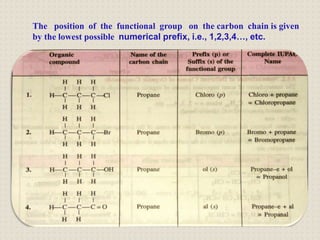
Carbon is a versatile element that forms millions of compounds. It exists in many forms including diamond and graphite. Carbon is present in all living organisms and is the main component of fuels like coal.
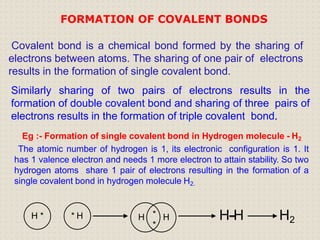
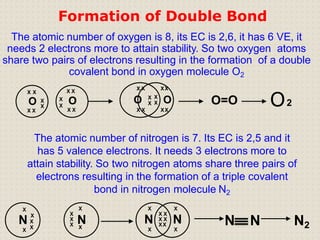
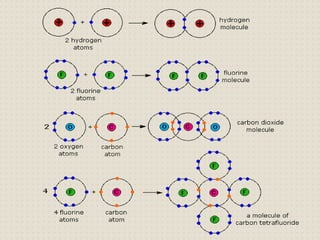
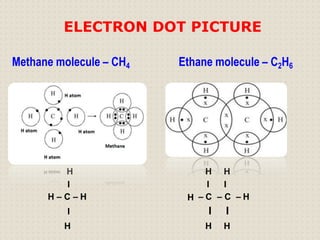
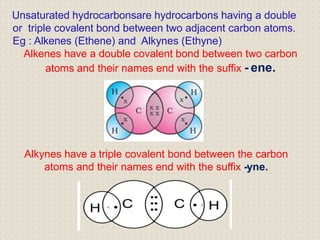
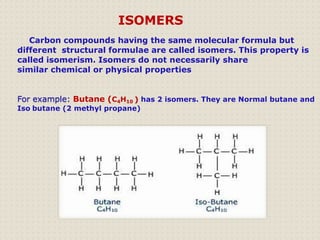
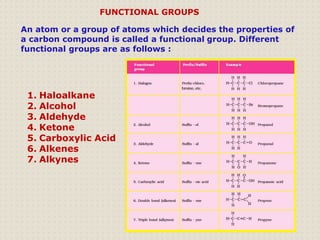
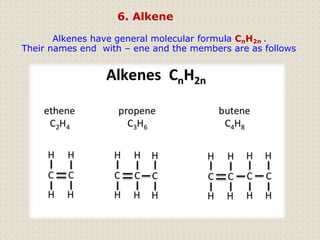
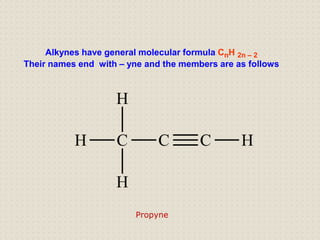
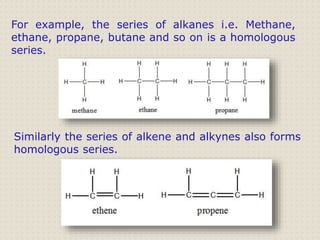
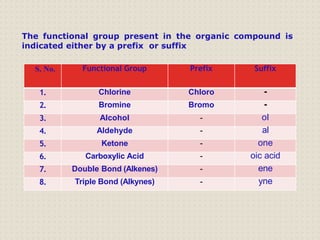
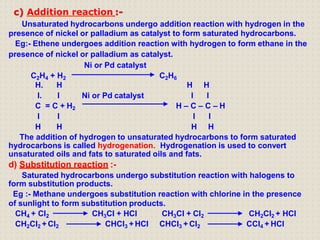
Carbon atoms bond with other atoms through covalent bonds by sharing electrons. This allows carbon to form chains, branches and closed rings. Hydrocarbons contain only carbon and hydrogen and can be saturated or unsaturated. Functional groups determine the properties of carbon compounds.
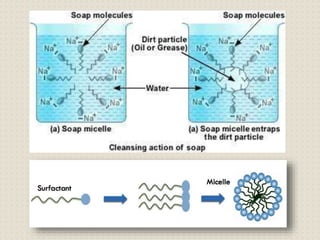
Some important carbon compounds are ethanol, ethanoic acid, and soaps. Ethanol is used in drinks and medicines while ethanoic acid gives vinegar its sour taste. Soaps clean through micelle formation while detergents work better in