This PPT is part 1 of Cancer biology which contains information about,
types of tumors
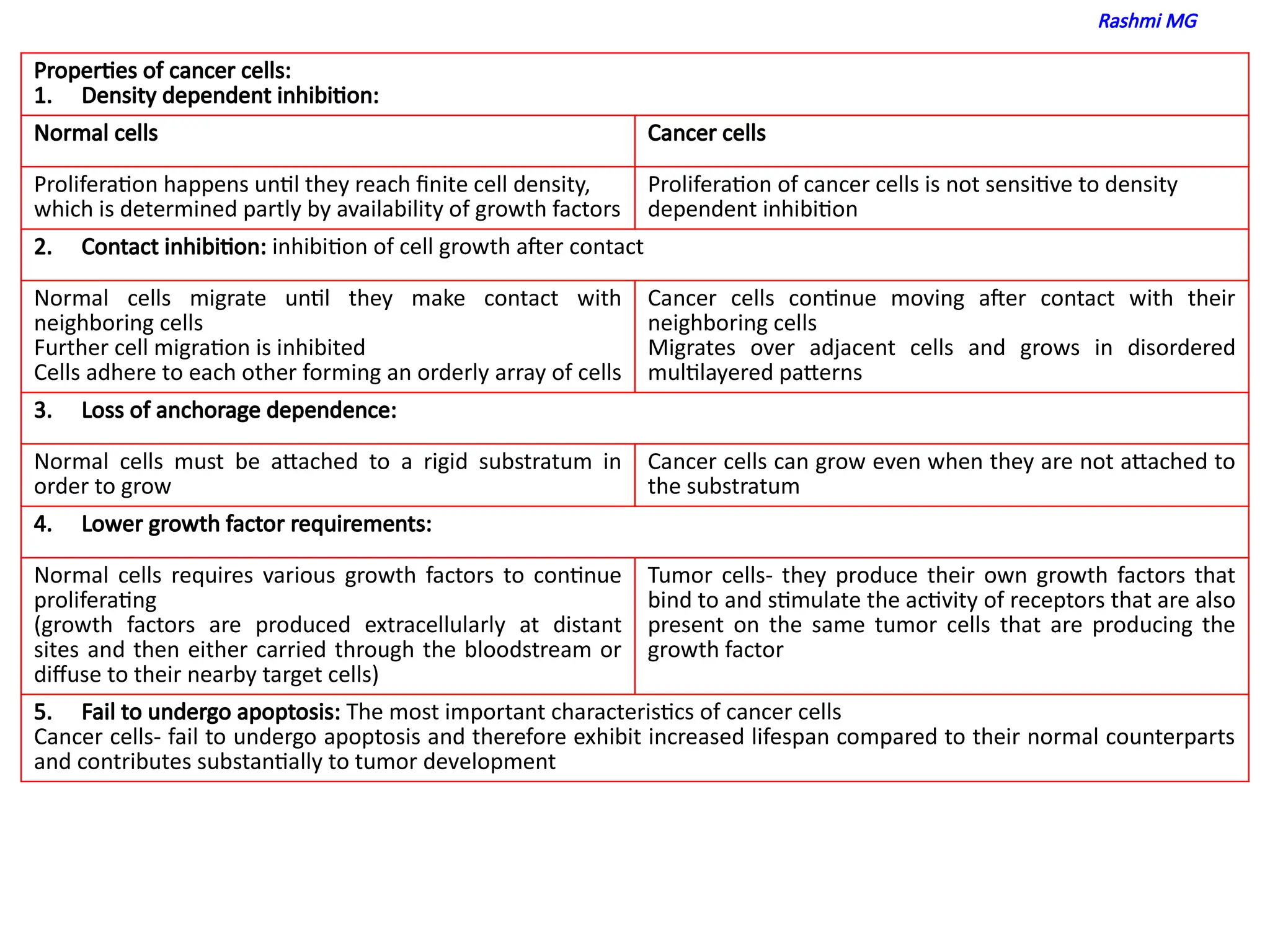
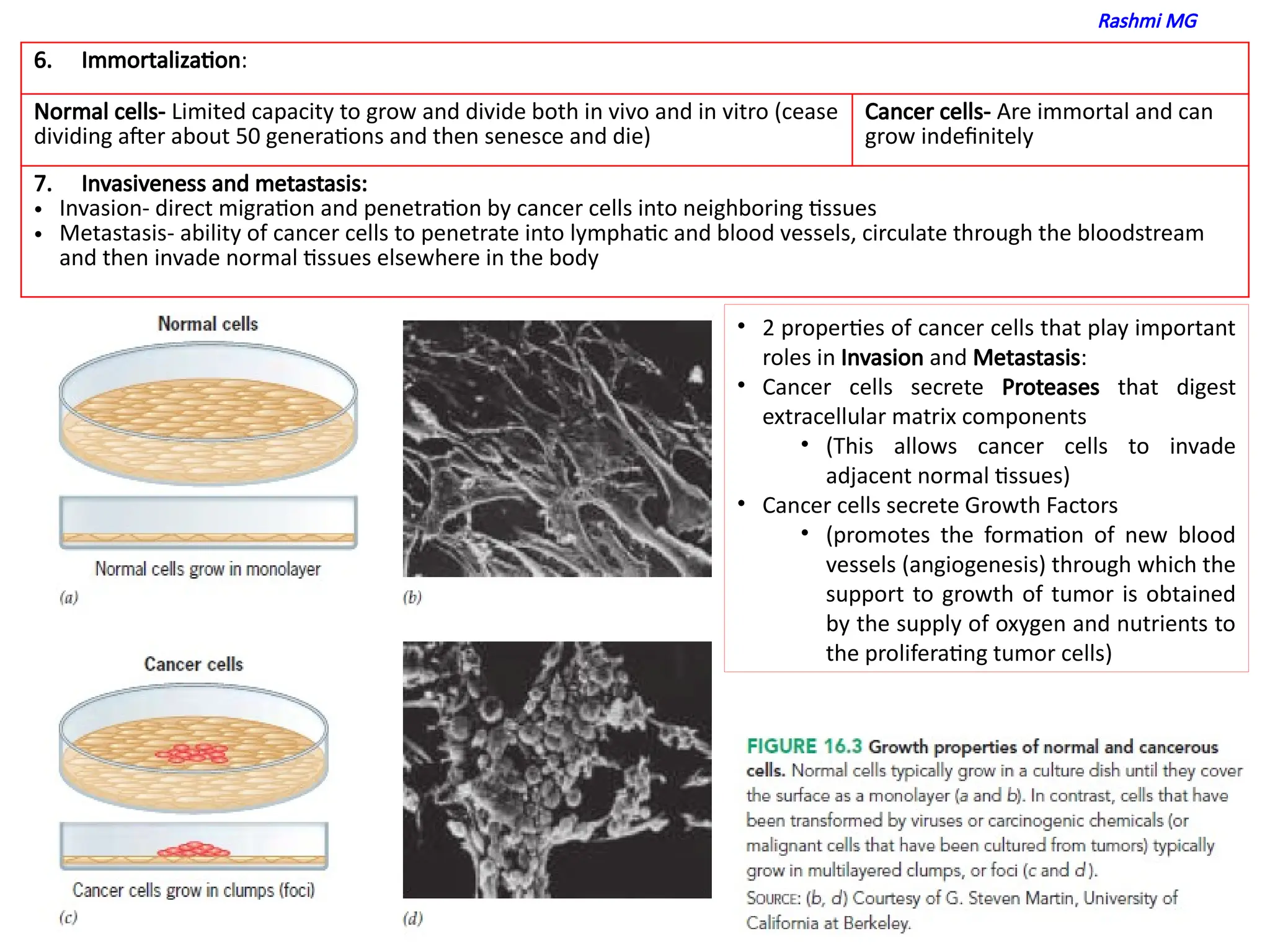
properties of cancer cells
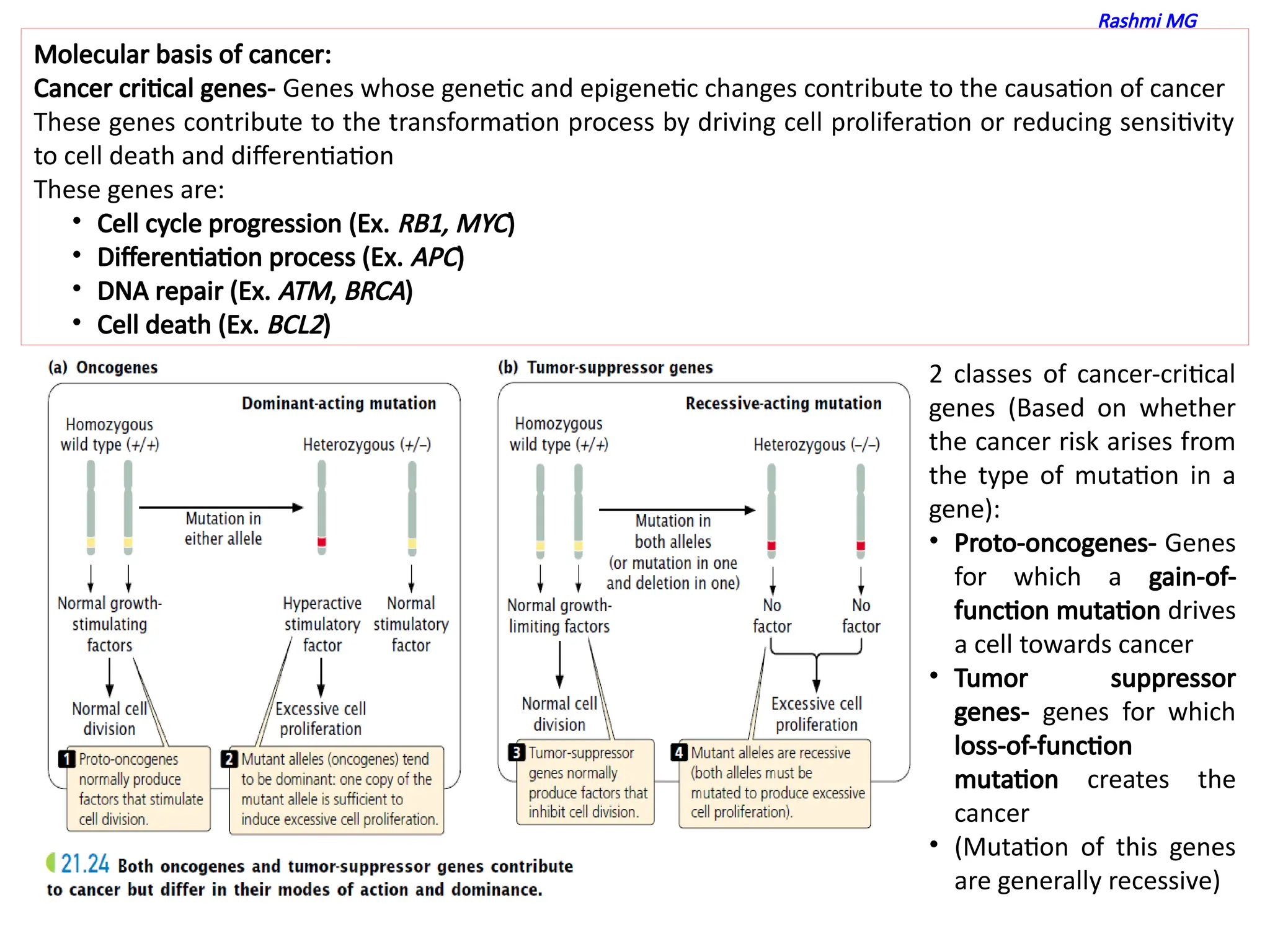
molecular basis of cancer
features of proto oncogenes
features of tumor suppressor genes
carcinogens- cancer causing agents
Importance of oncoviruses
features retroviral oncogenes