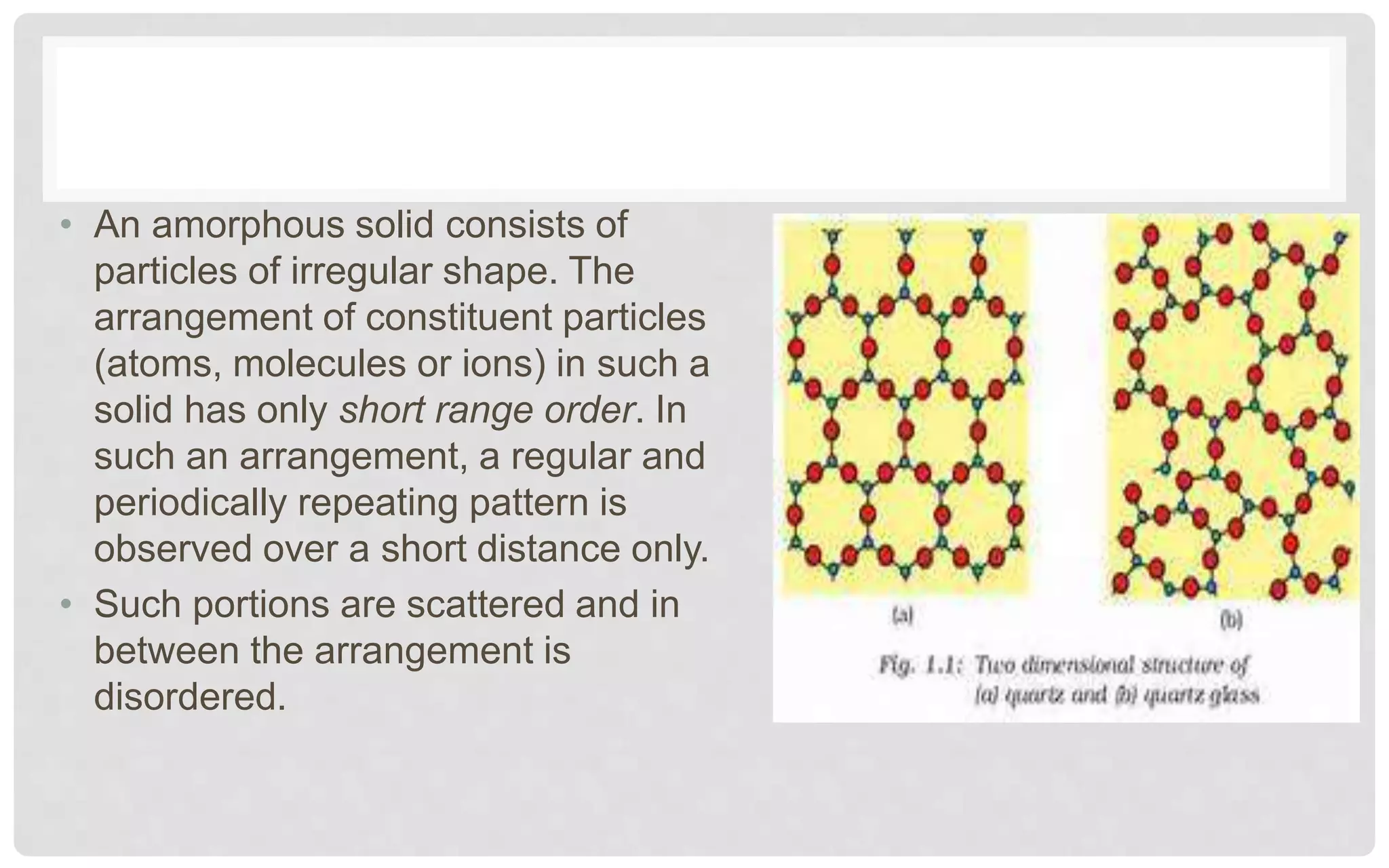
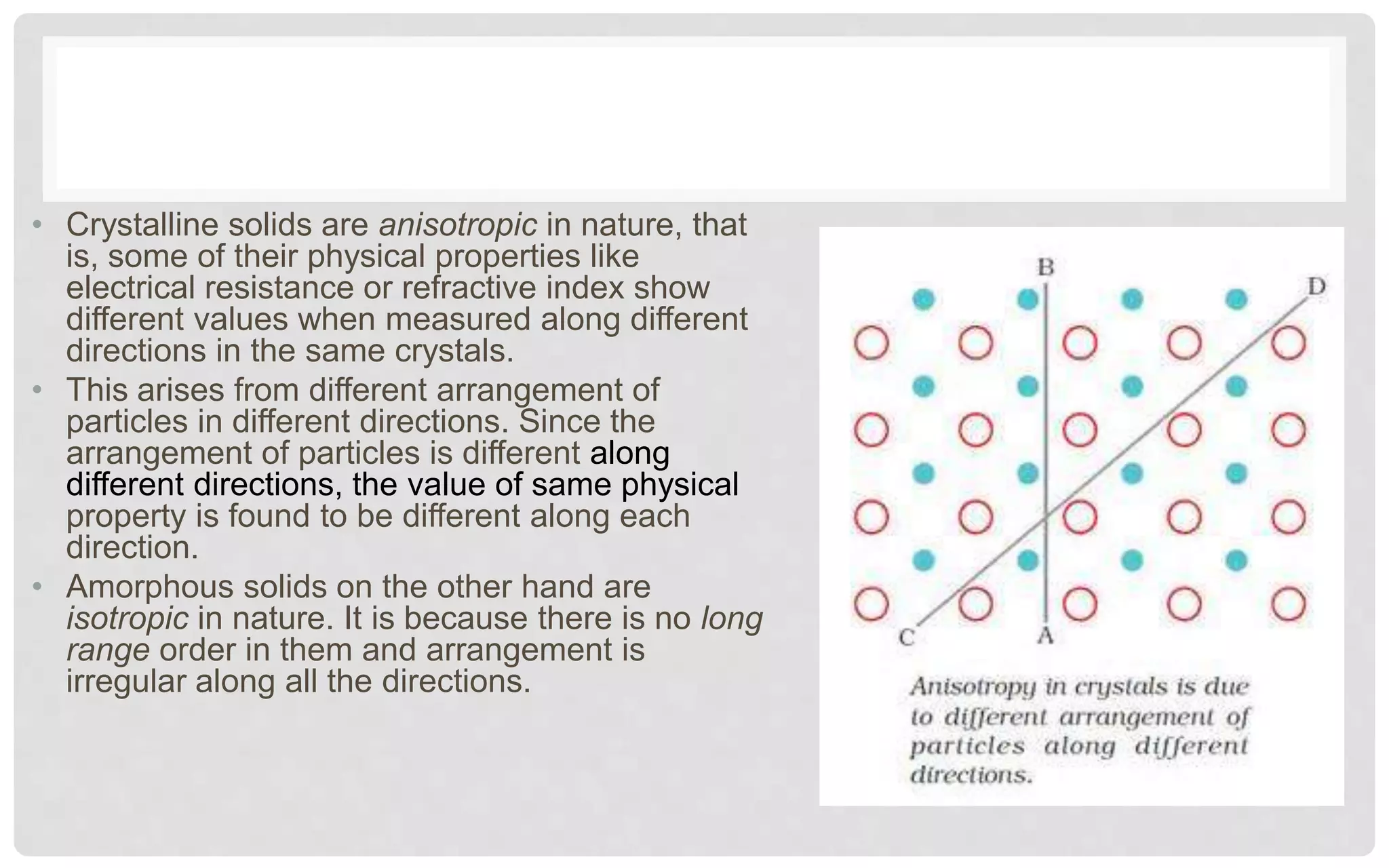
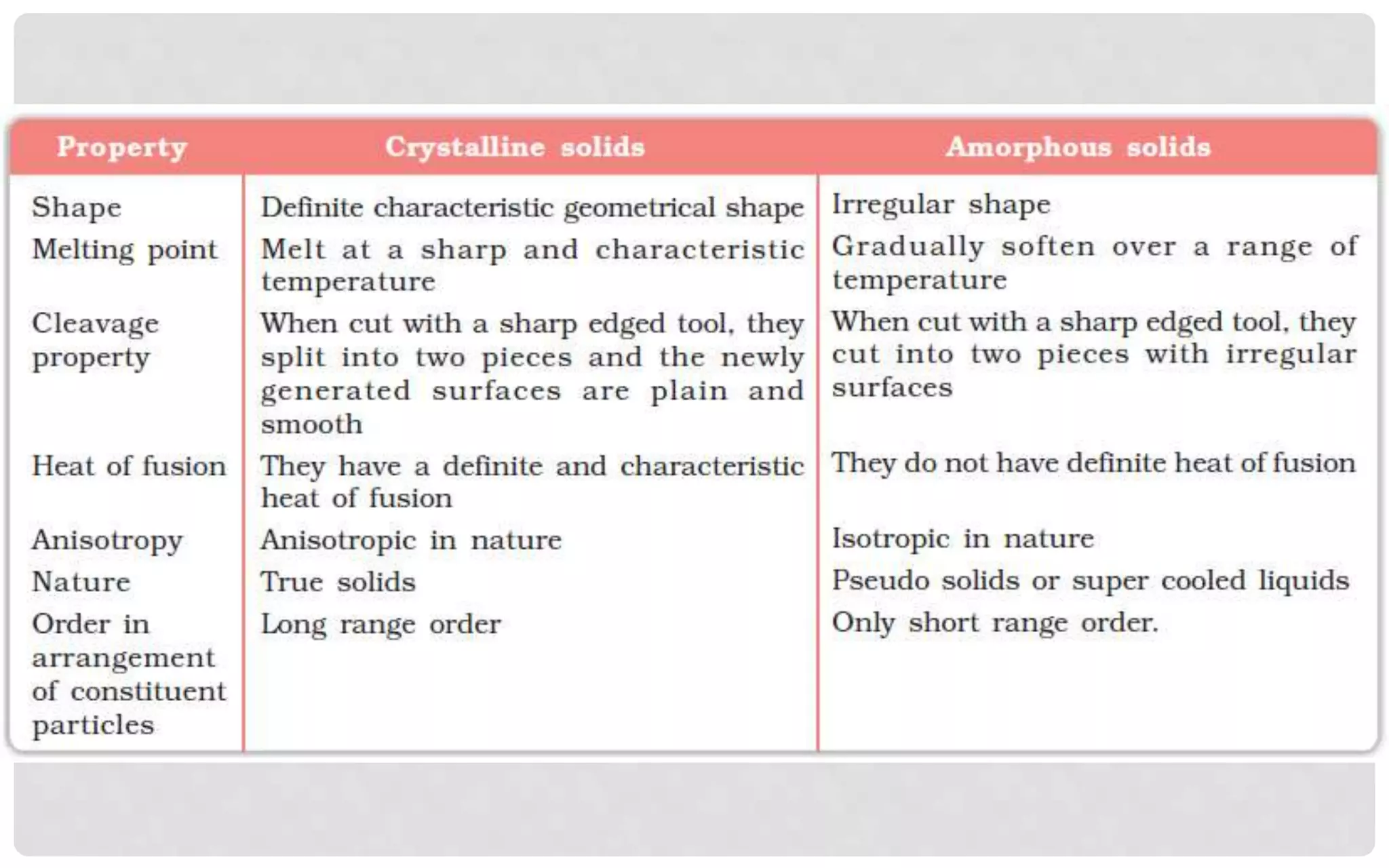
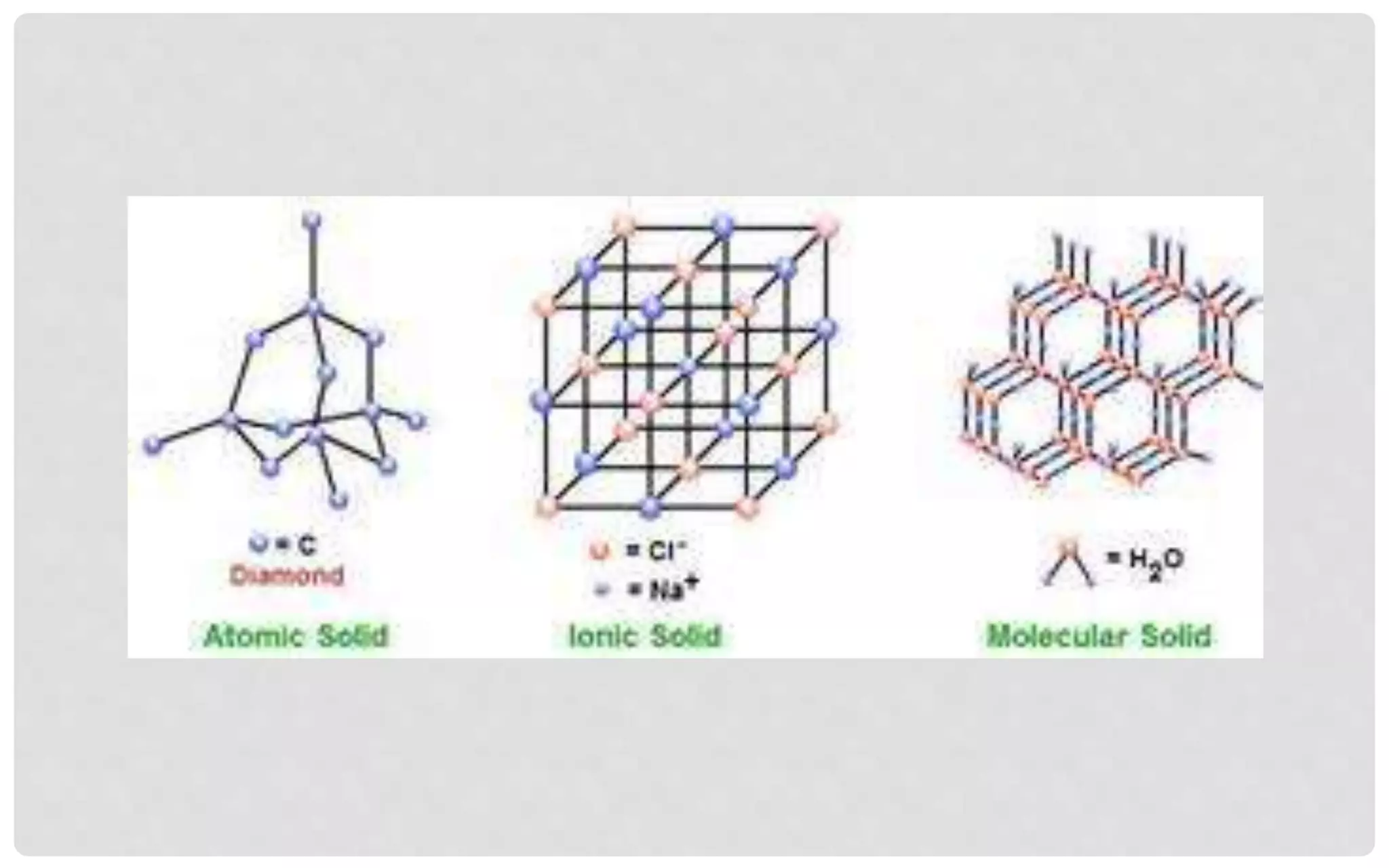
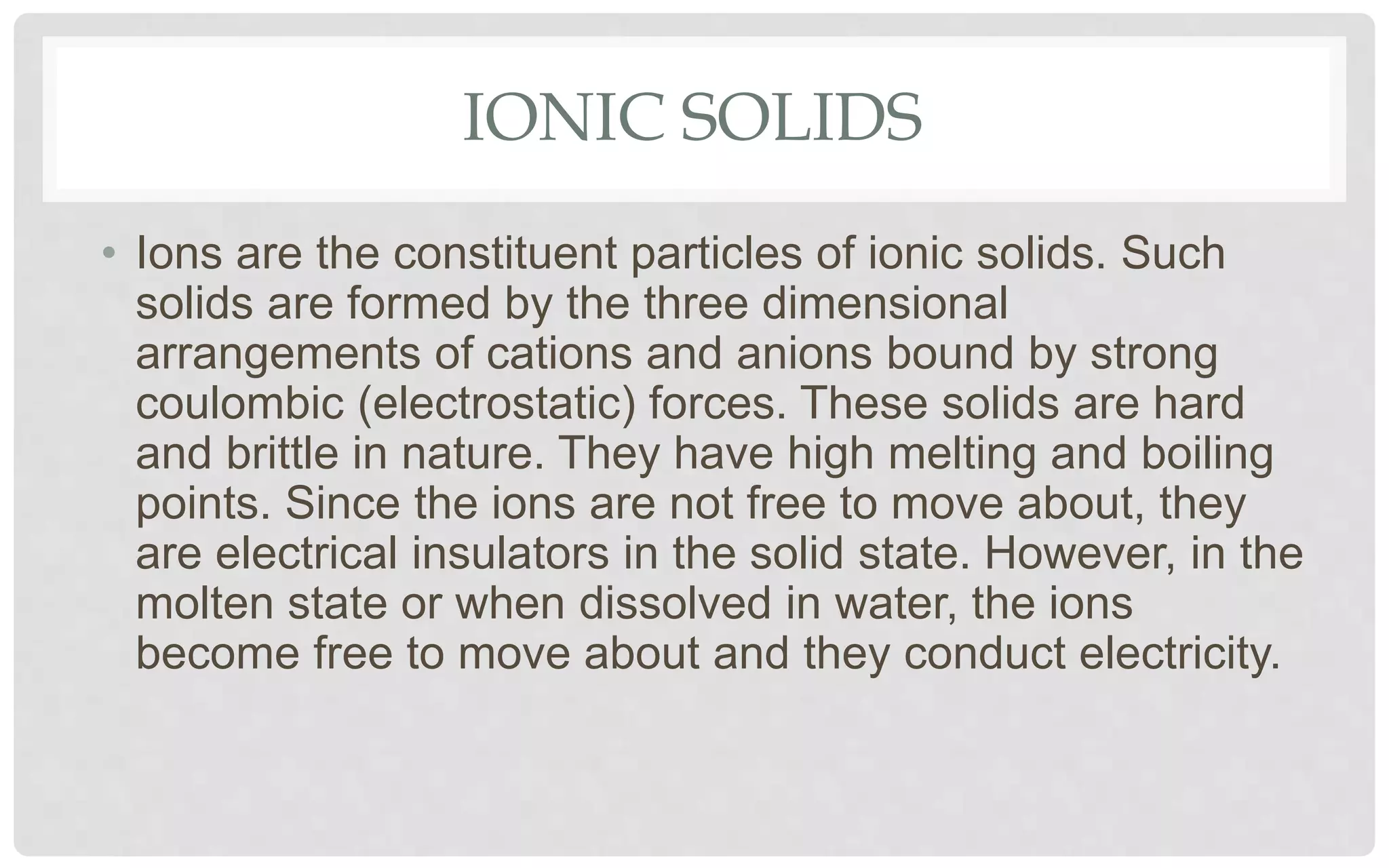
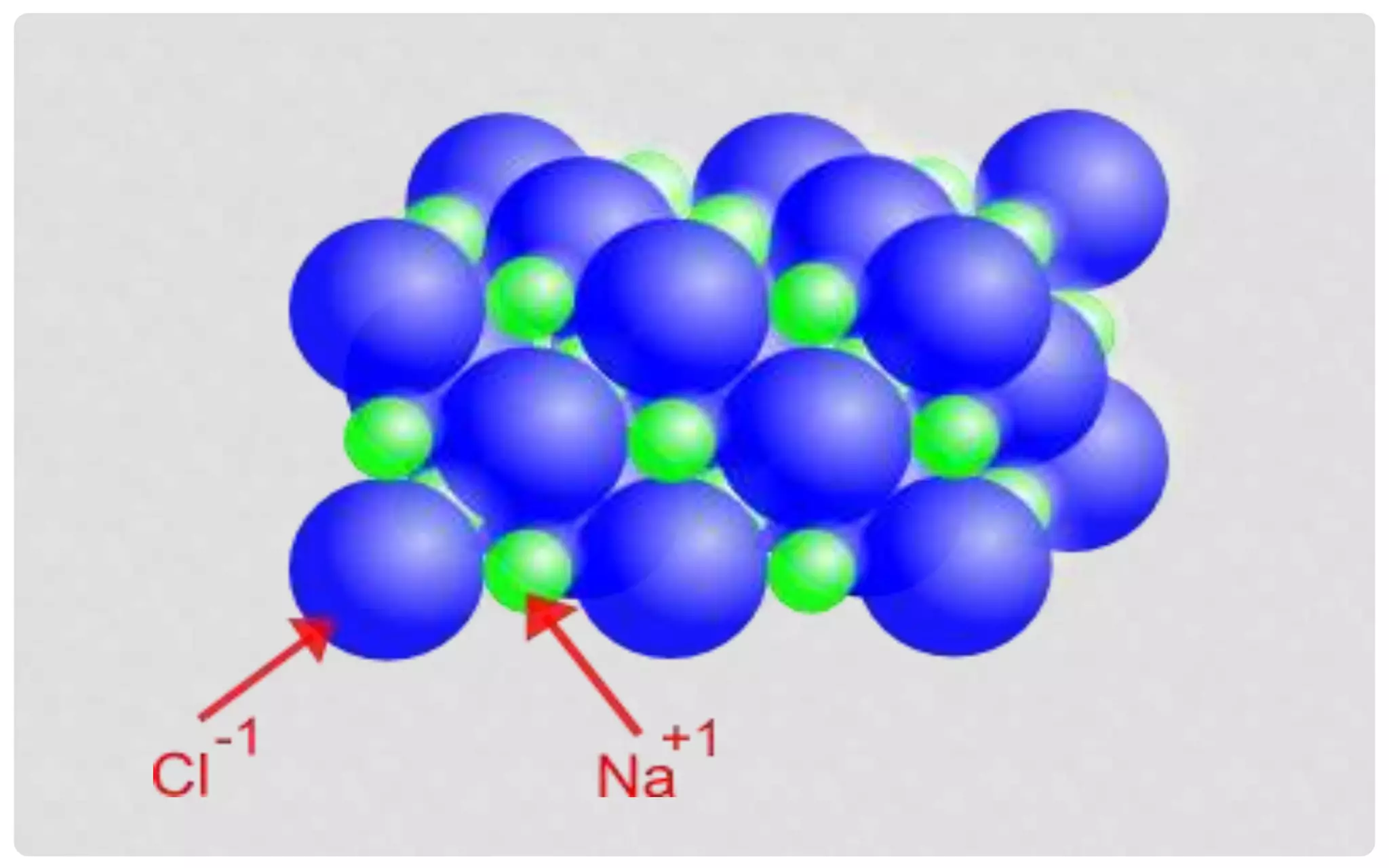
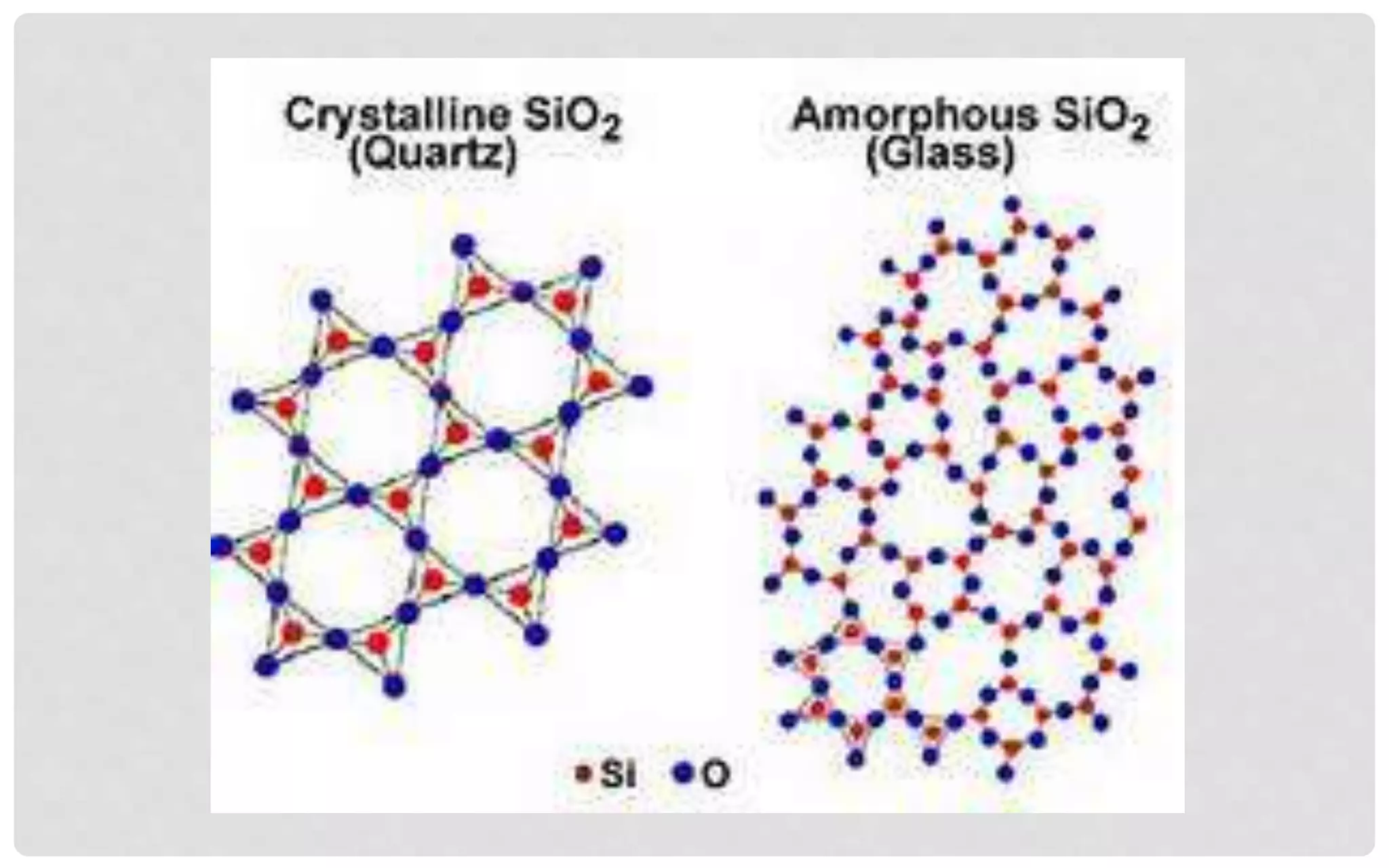
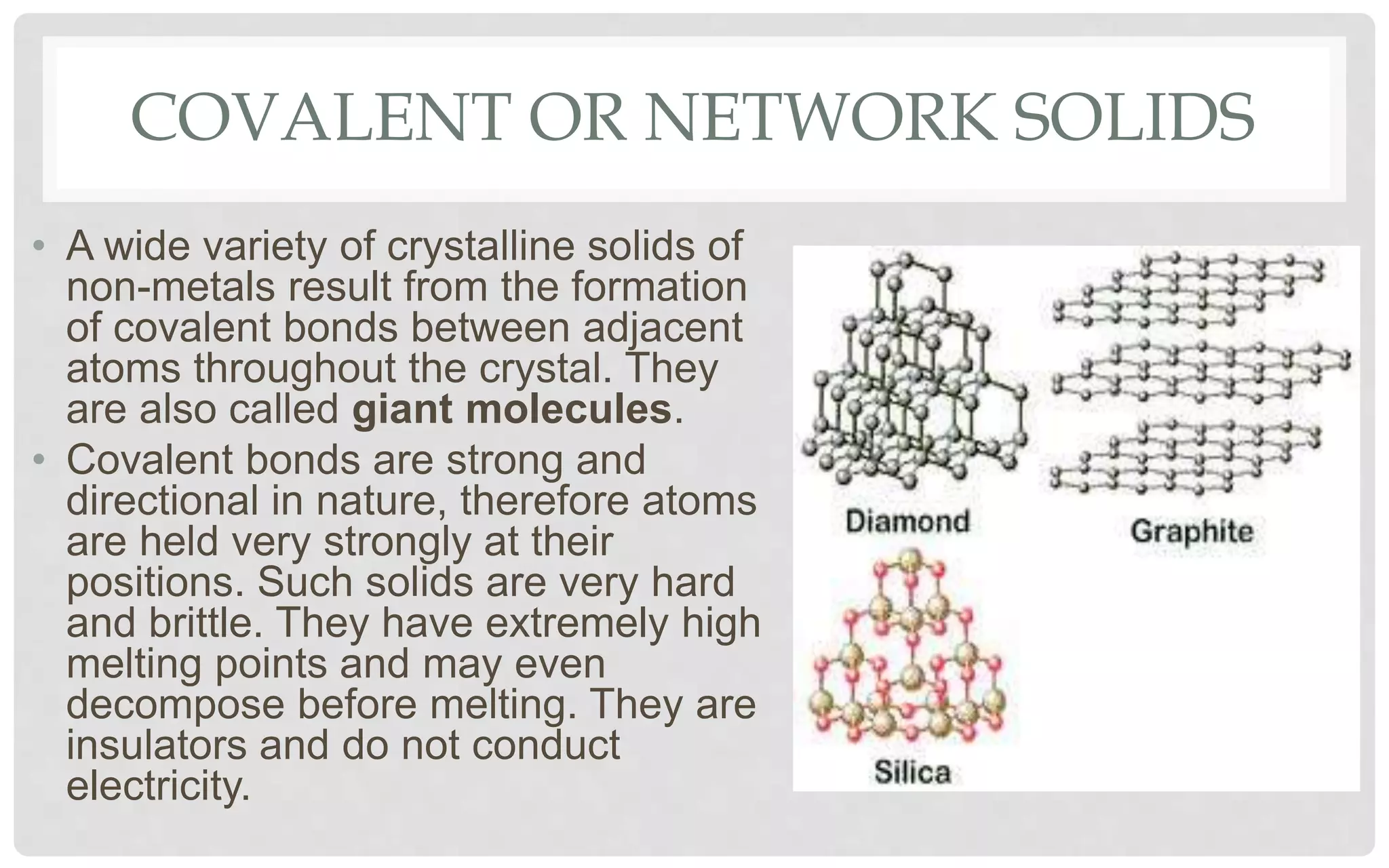
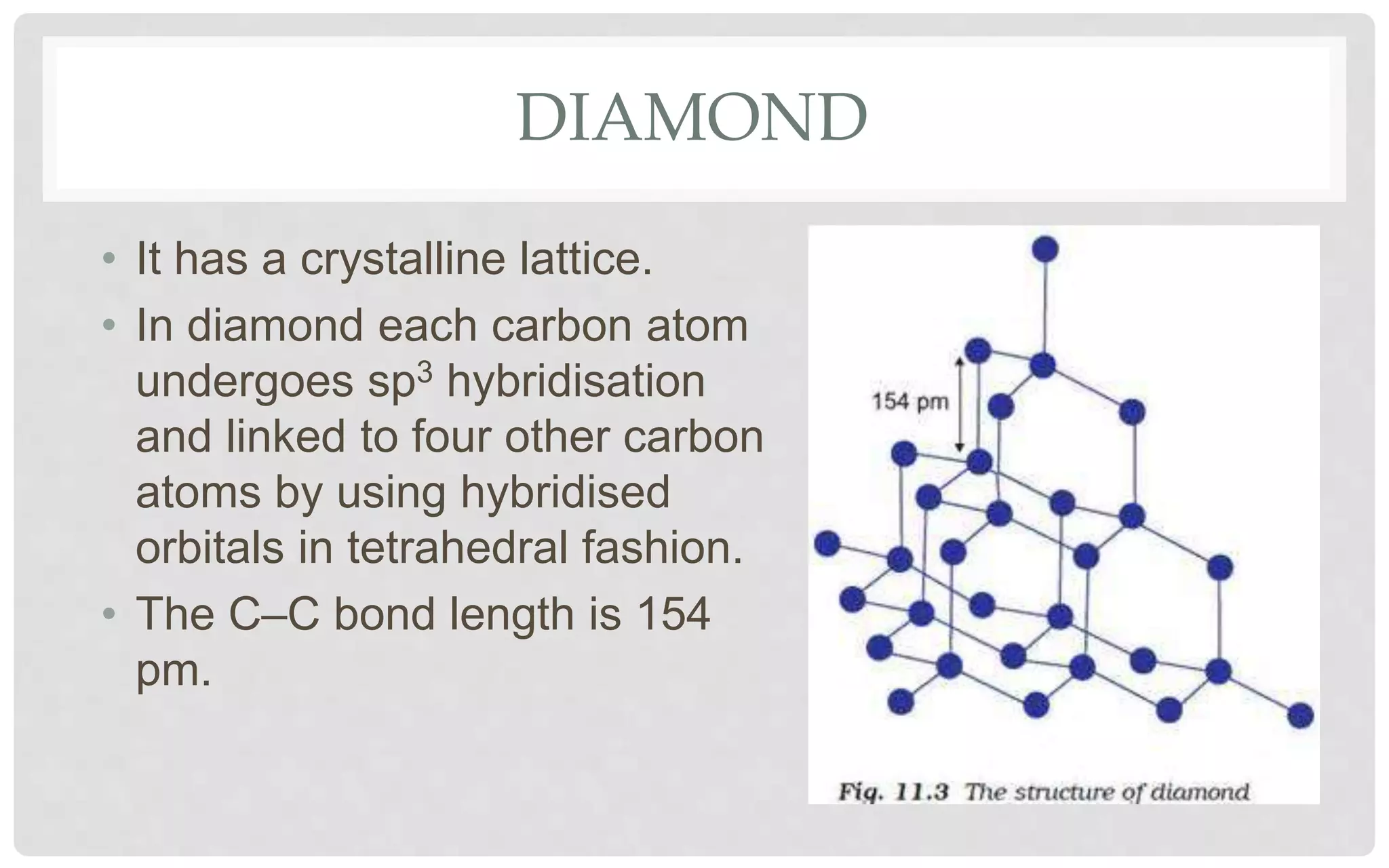
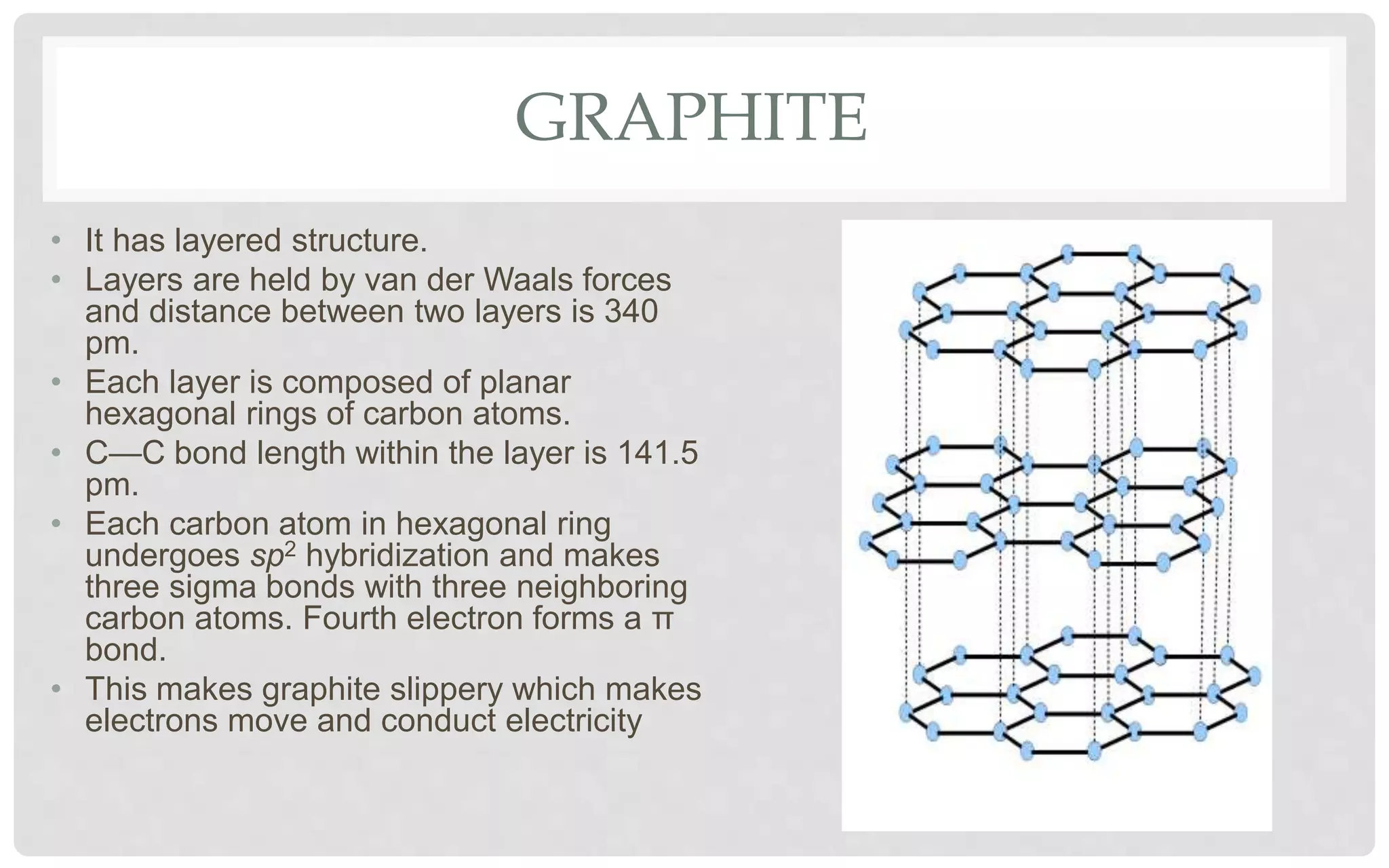
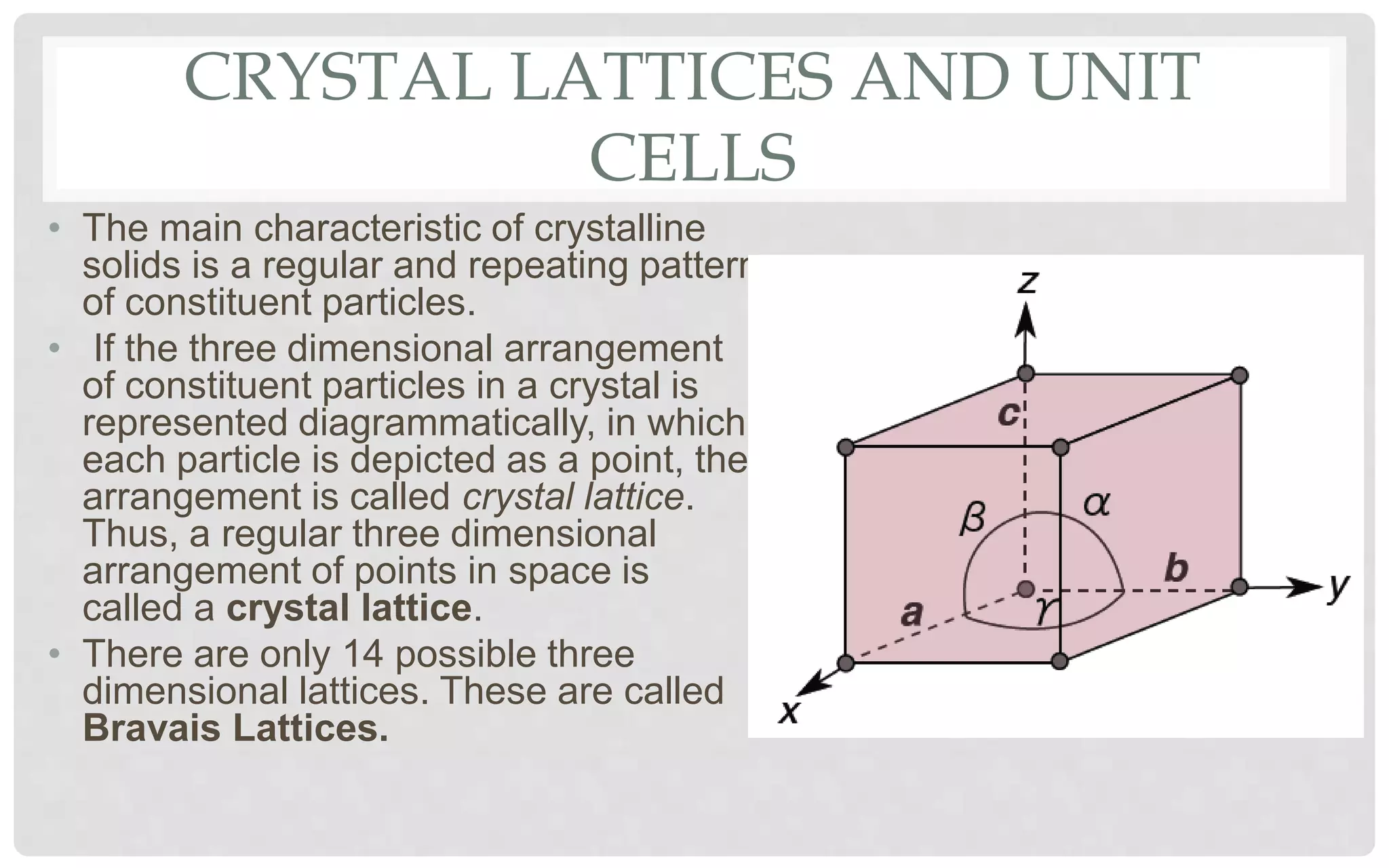
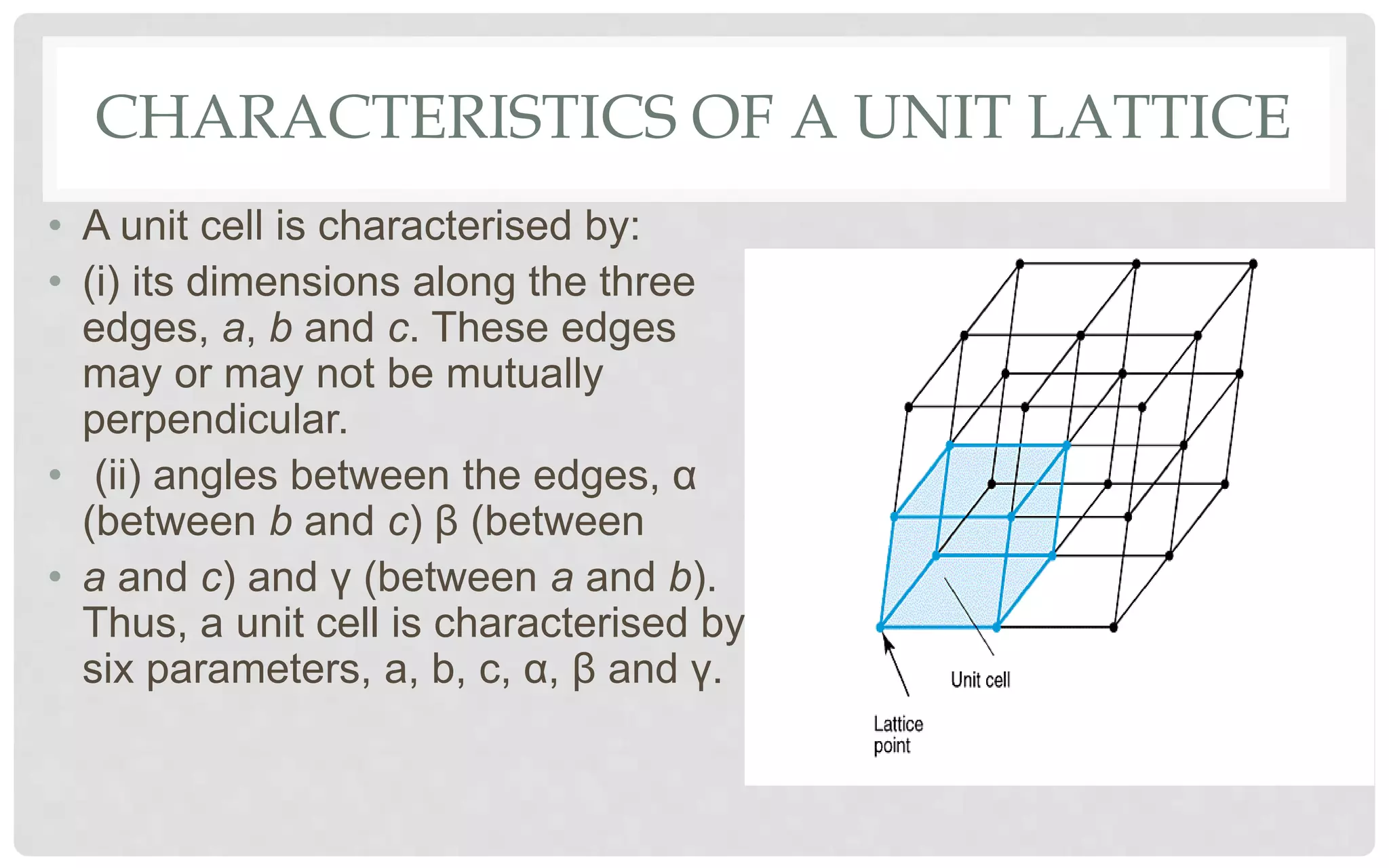
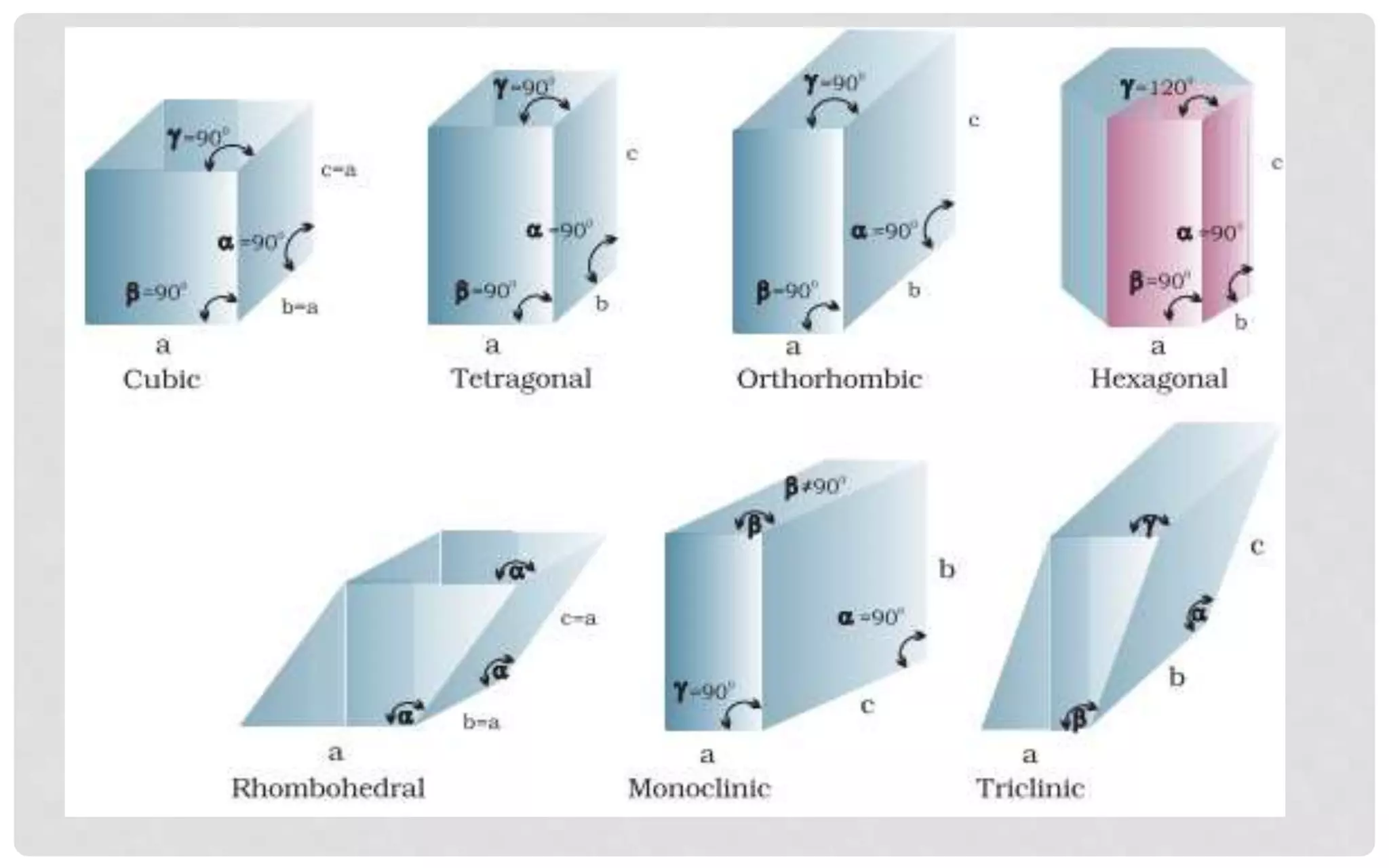
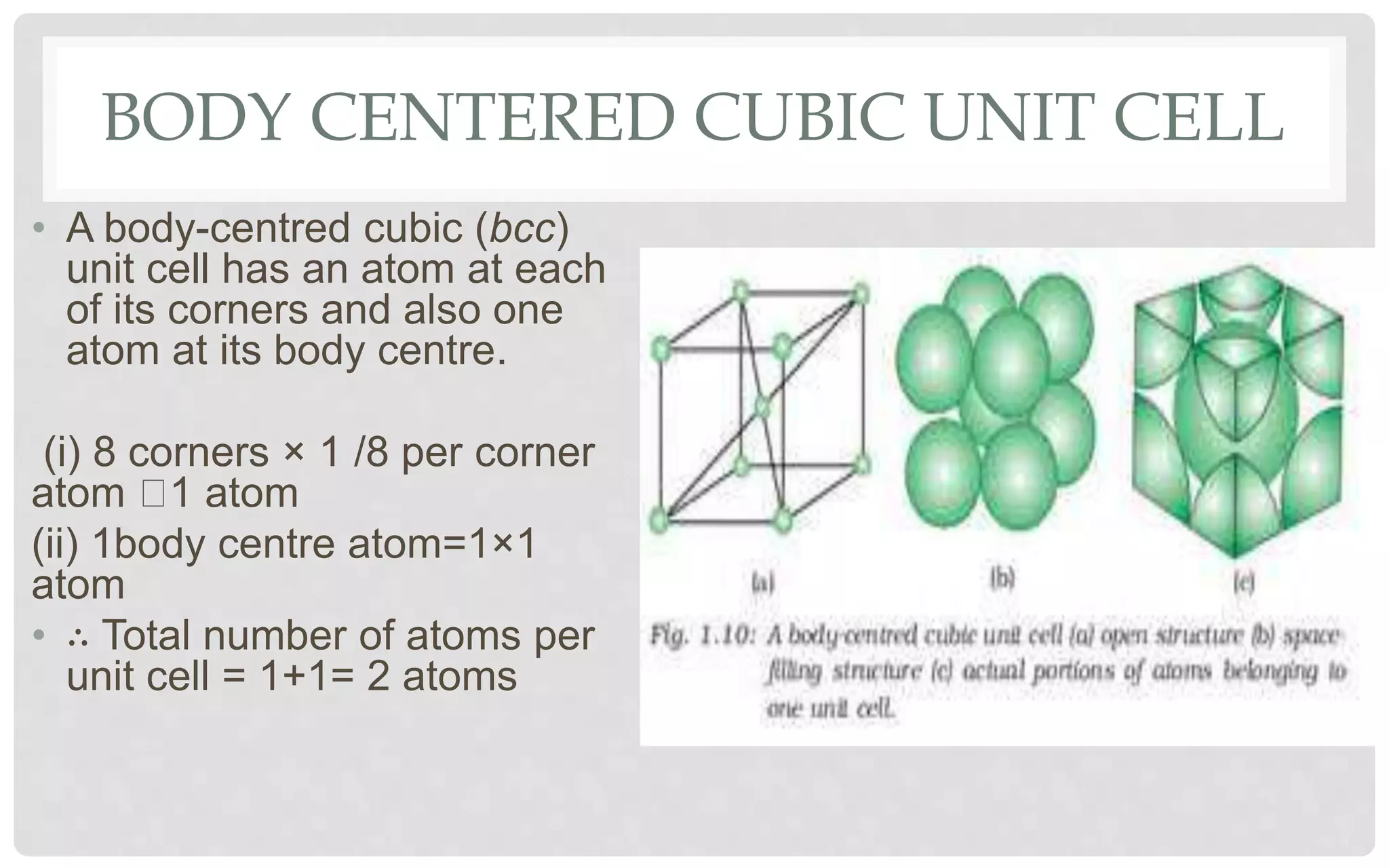
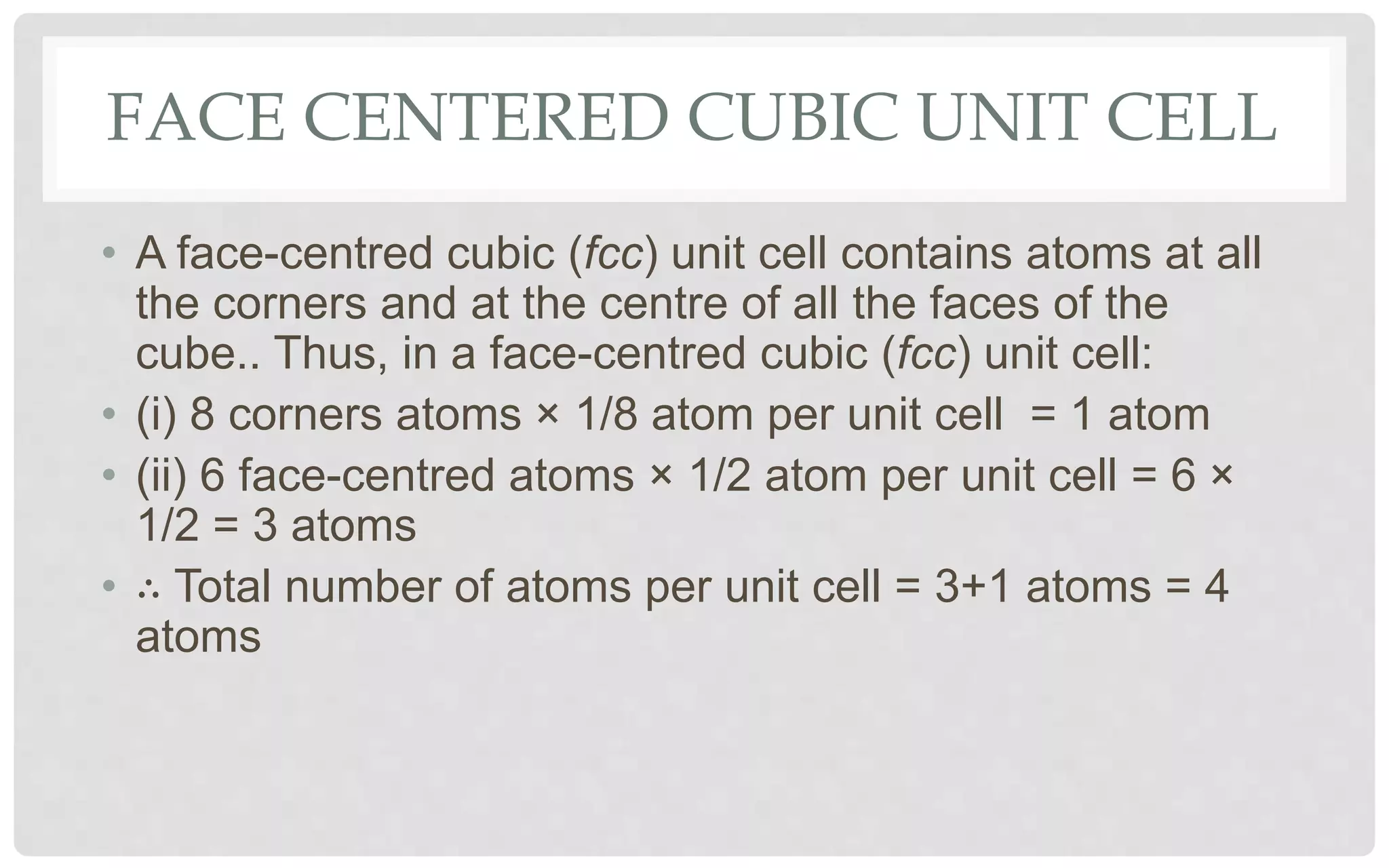
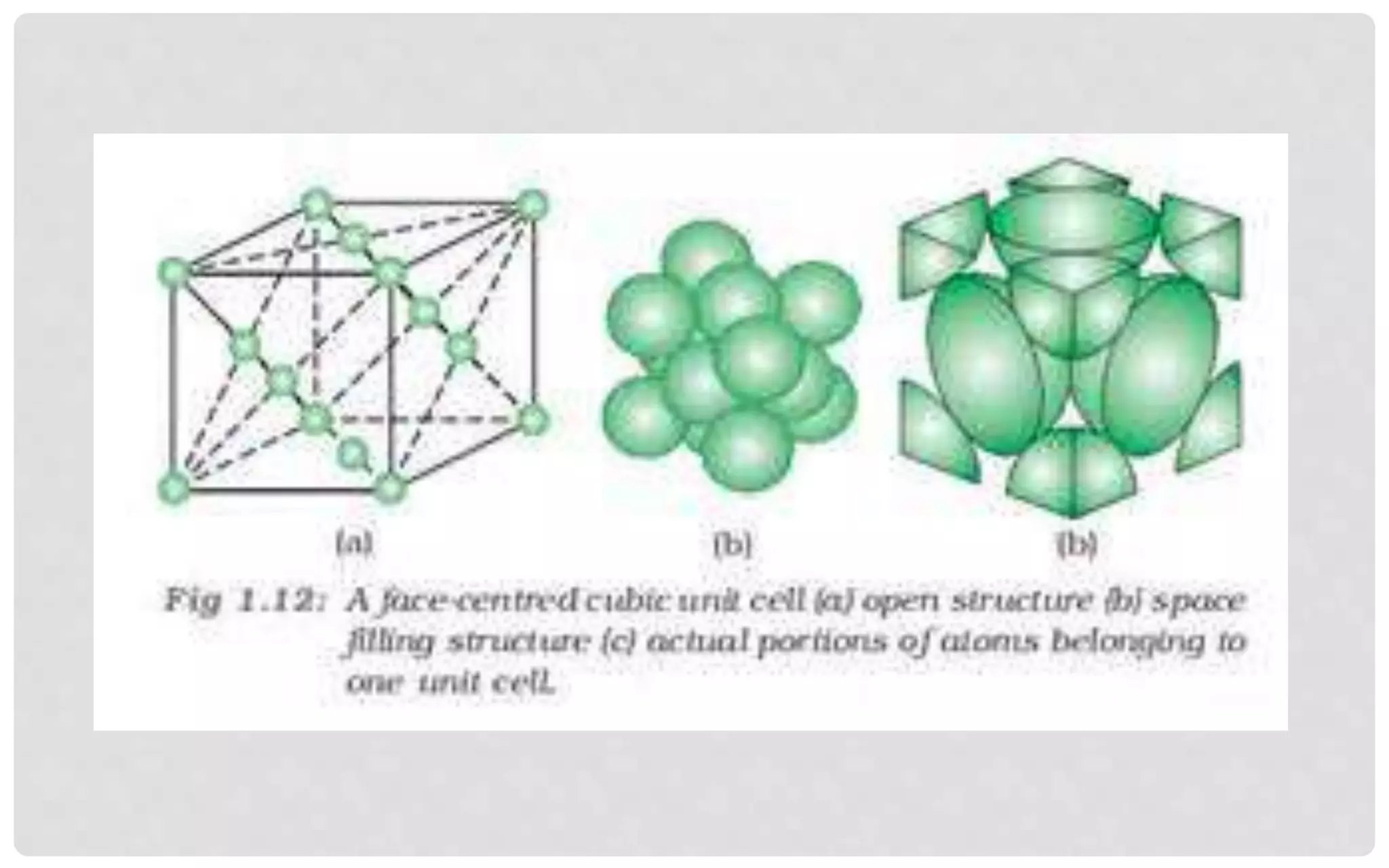
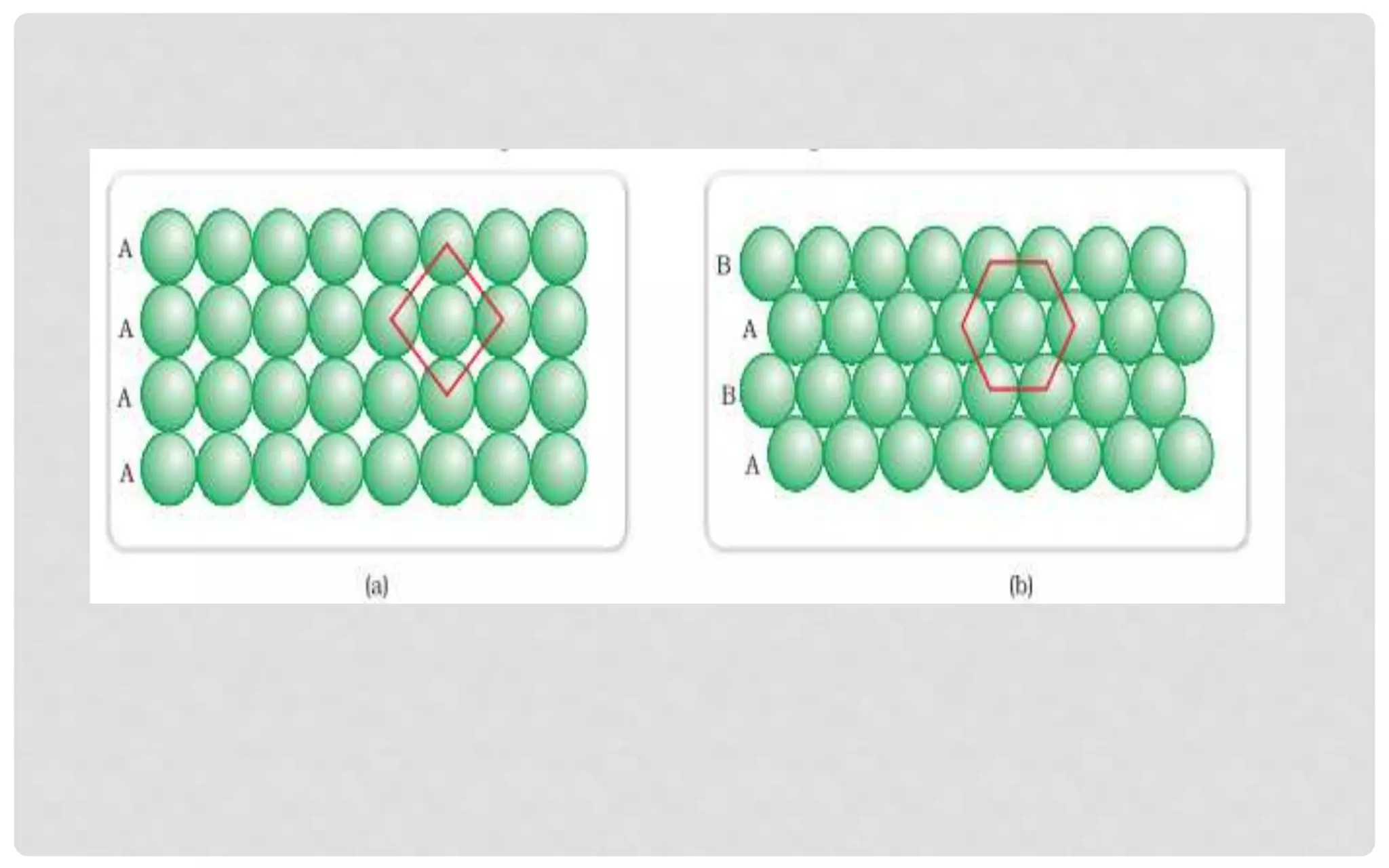
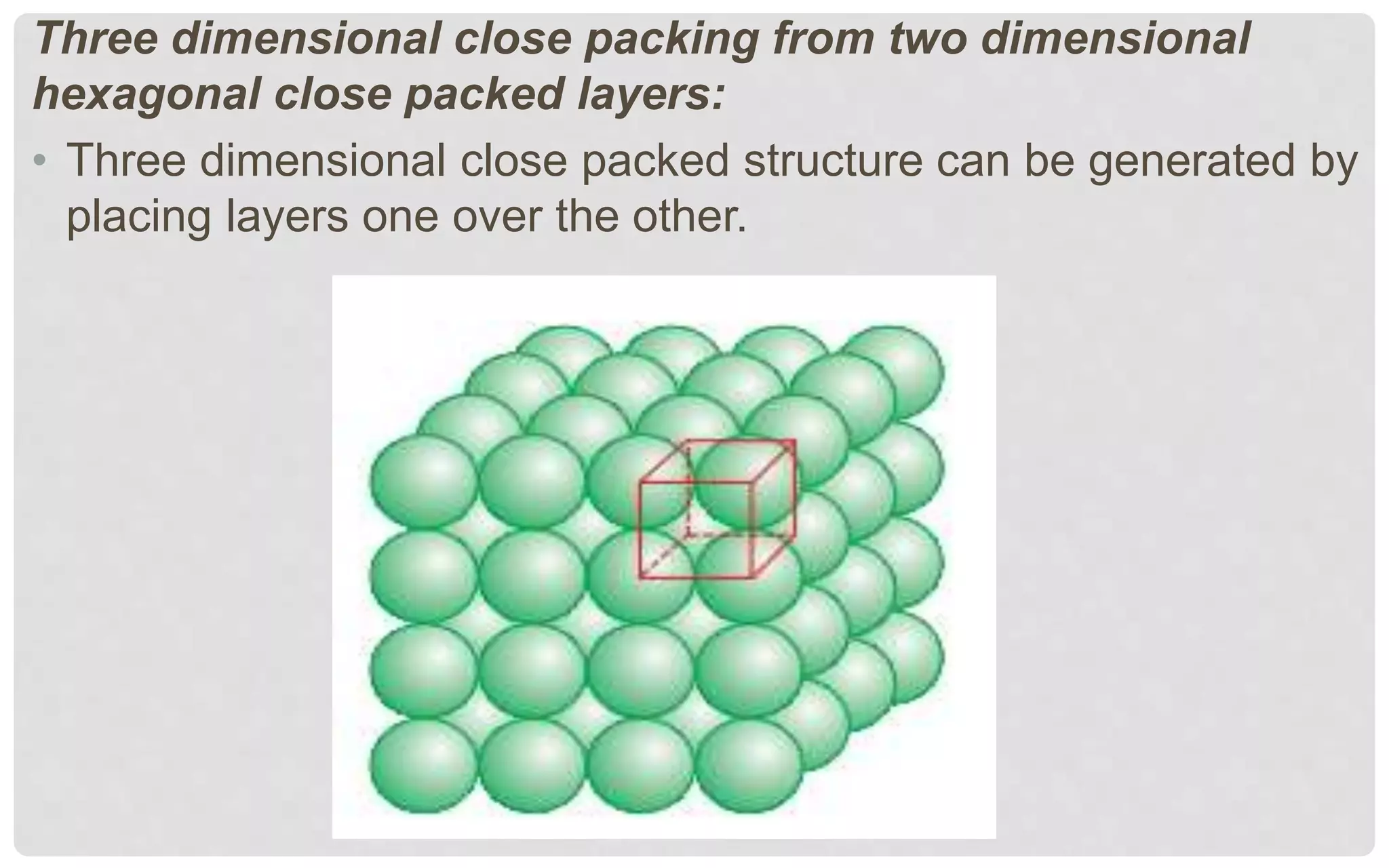
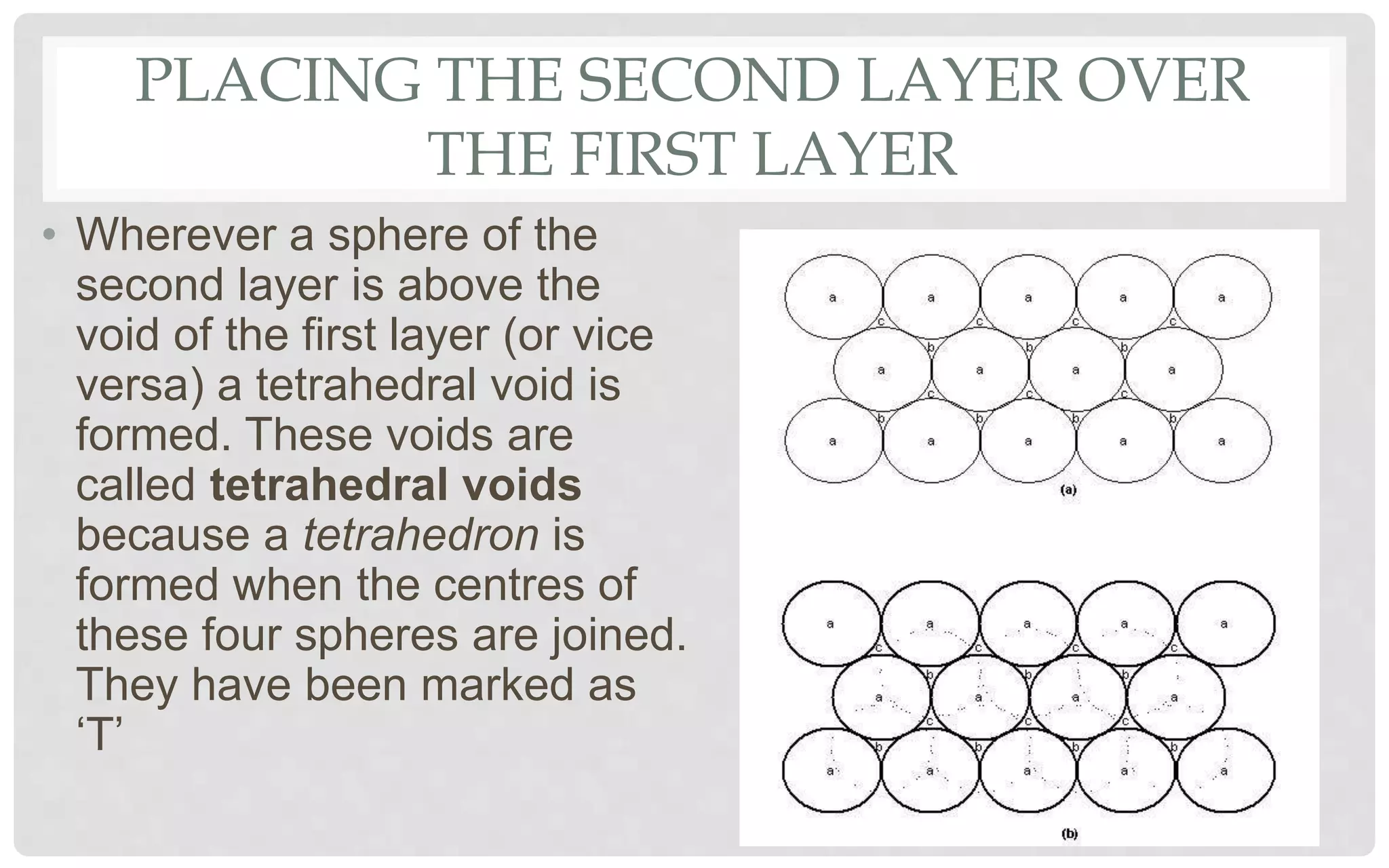
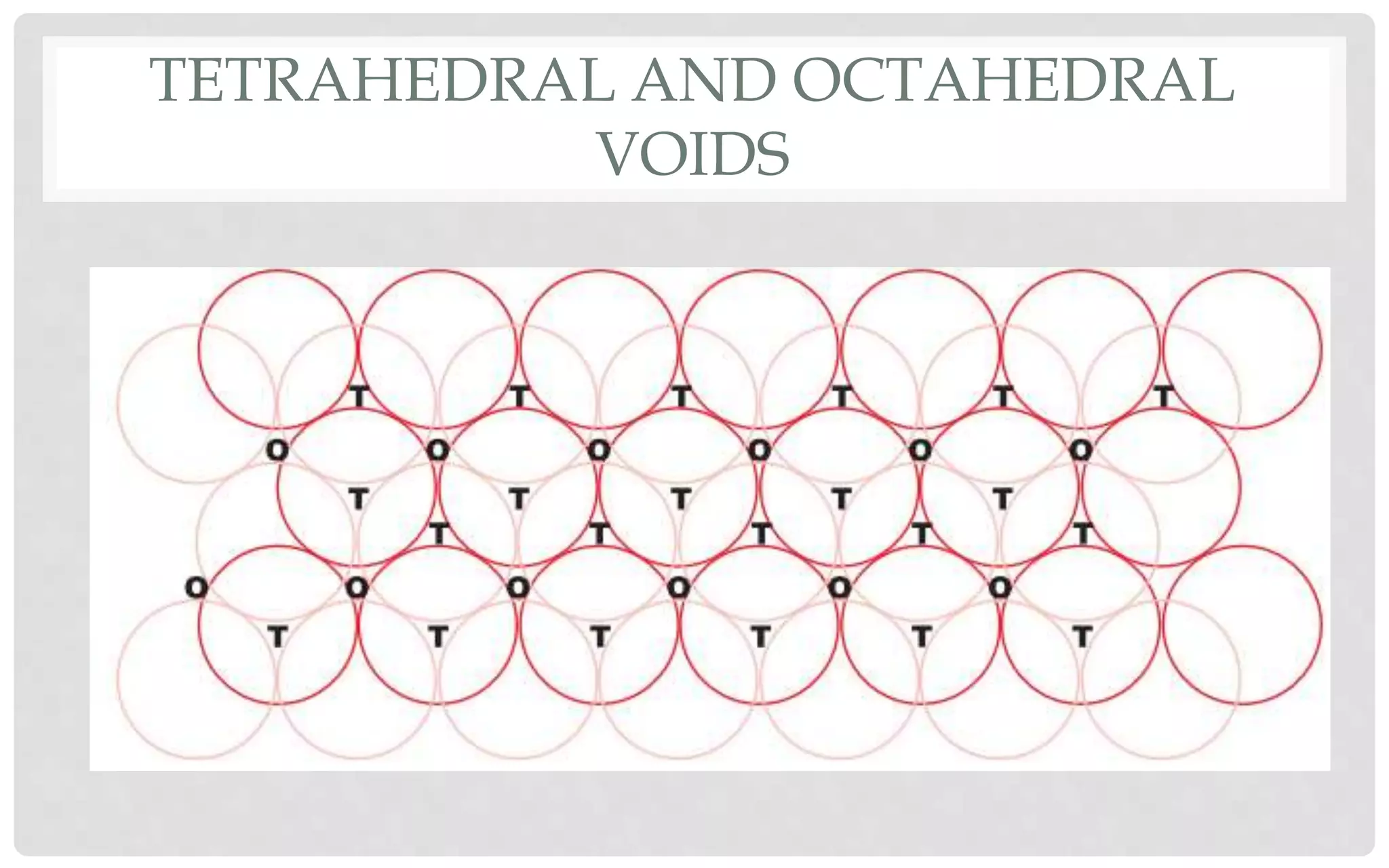
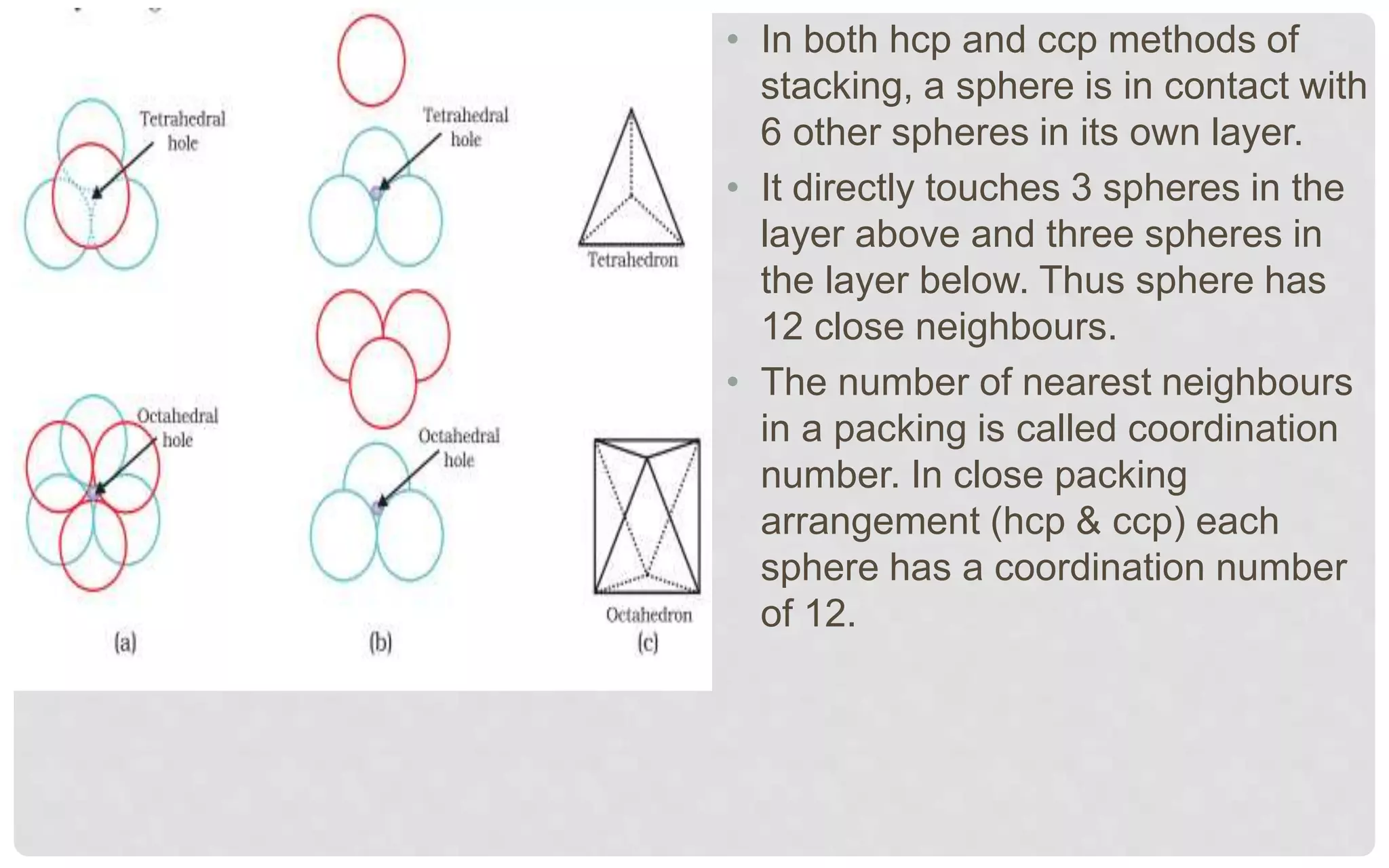
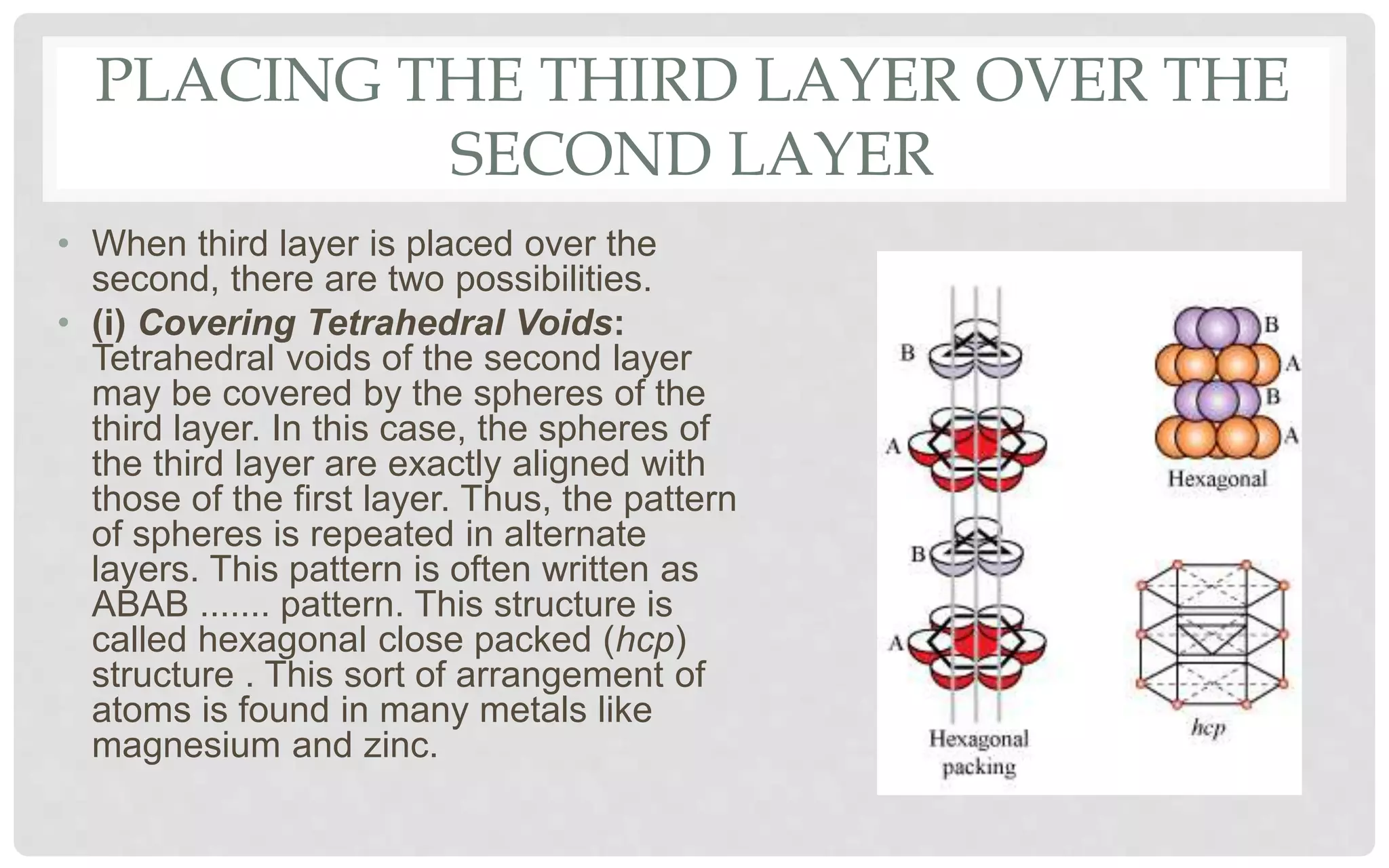
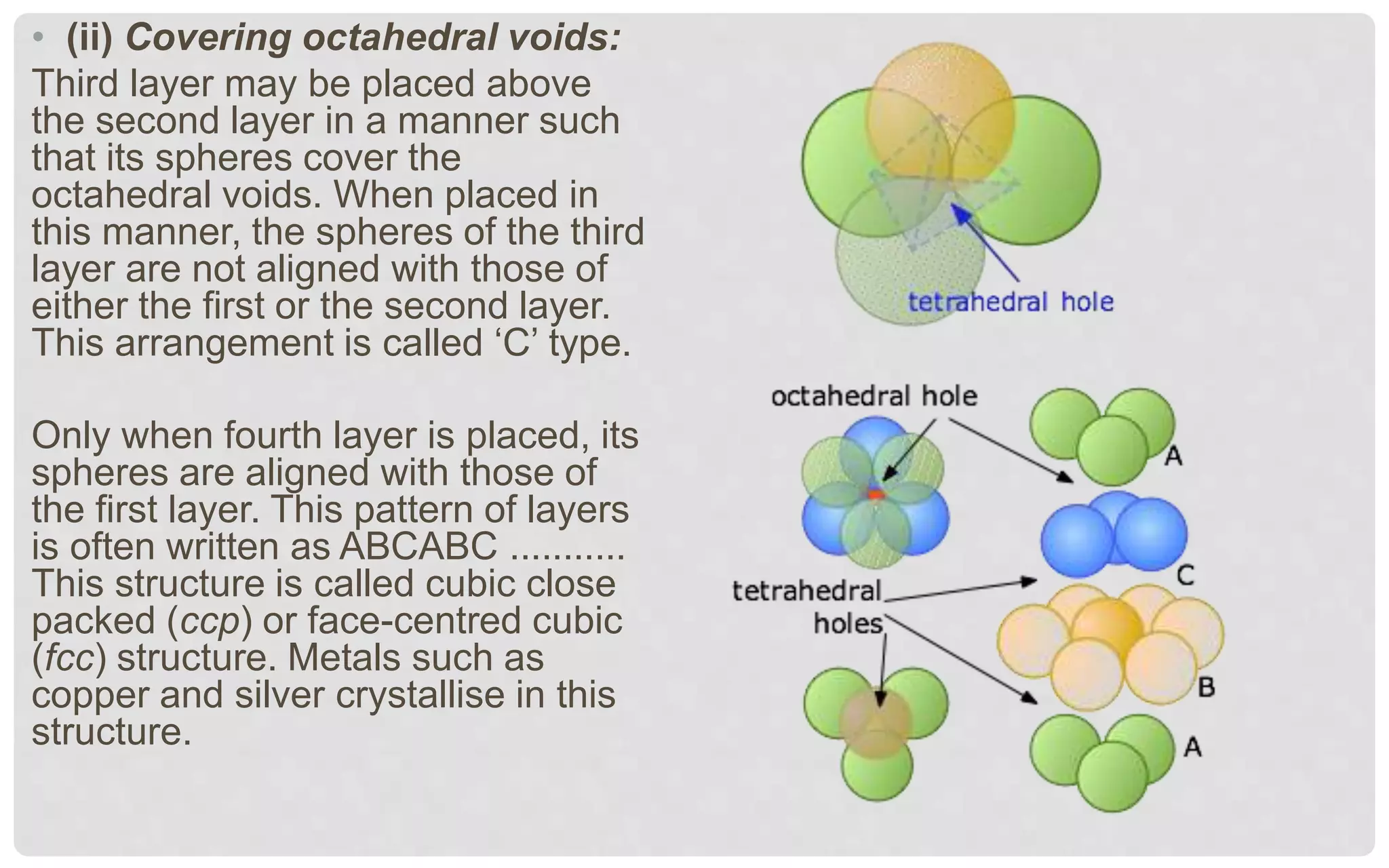
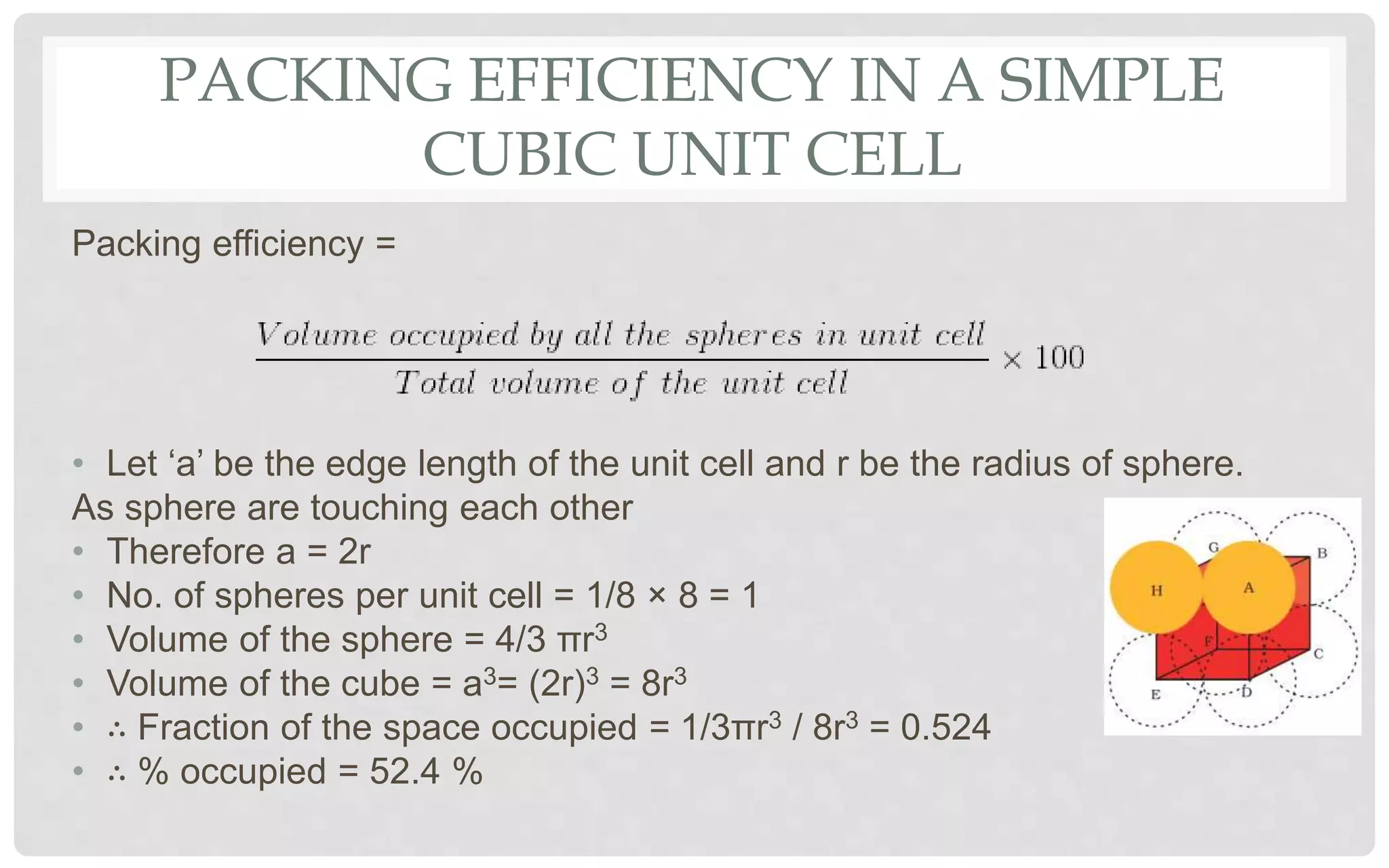
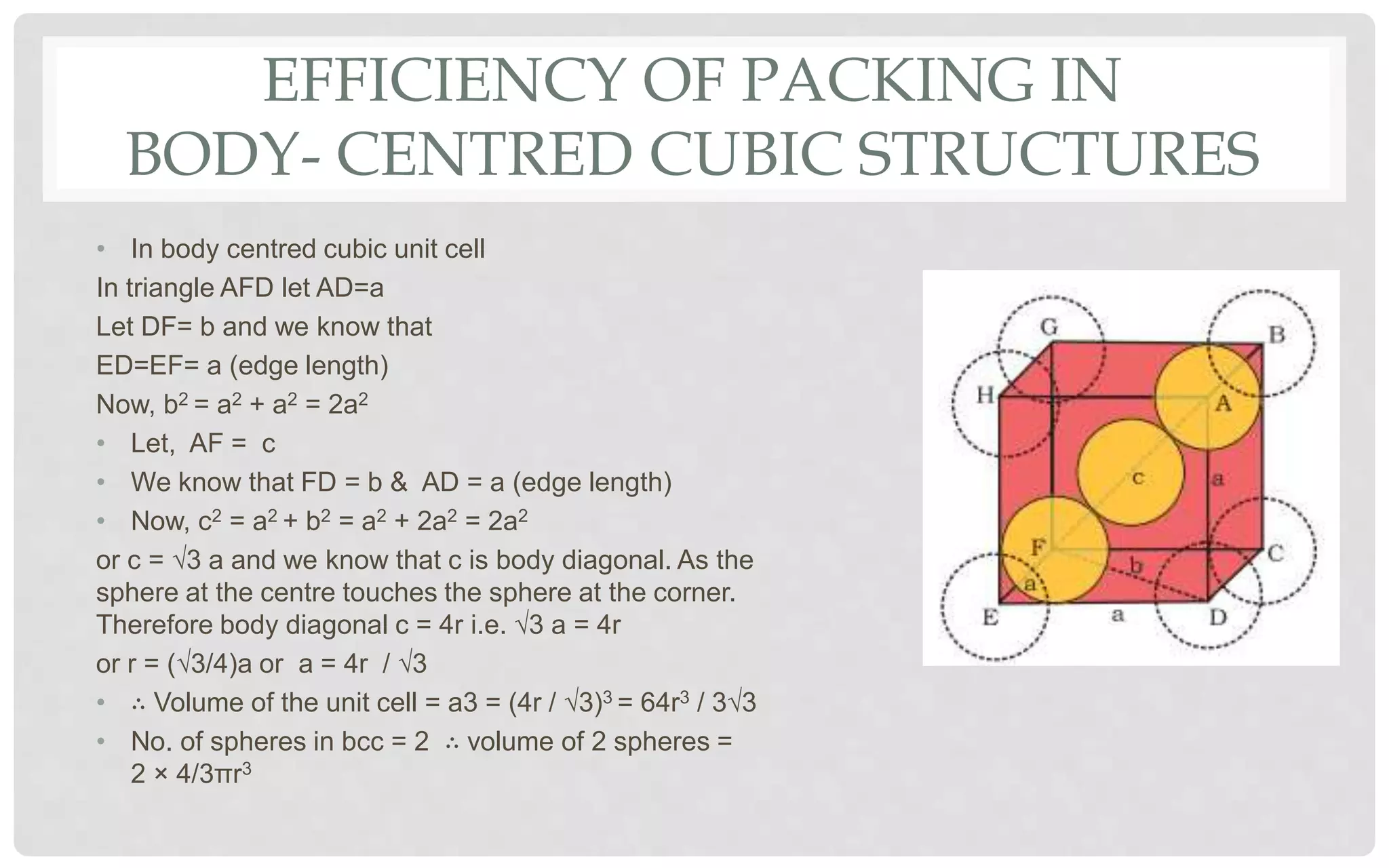
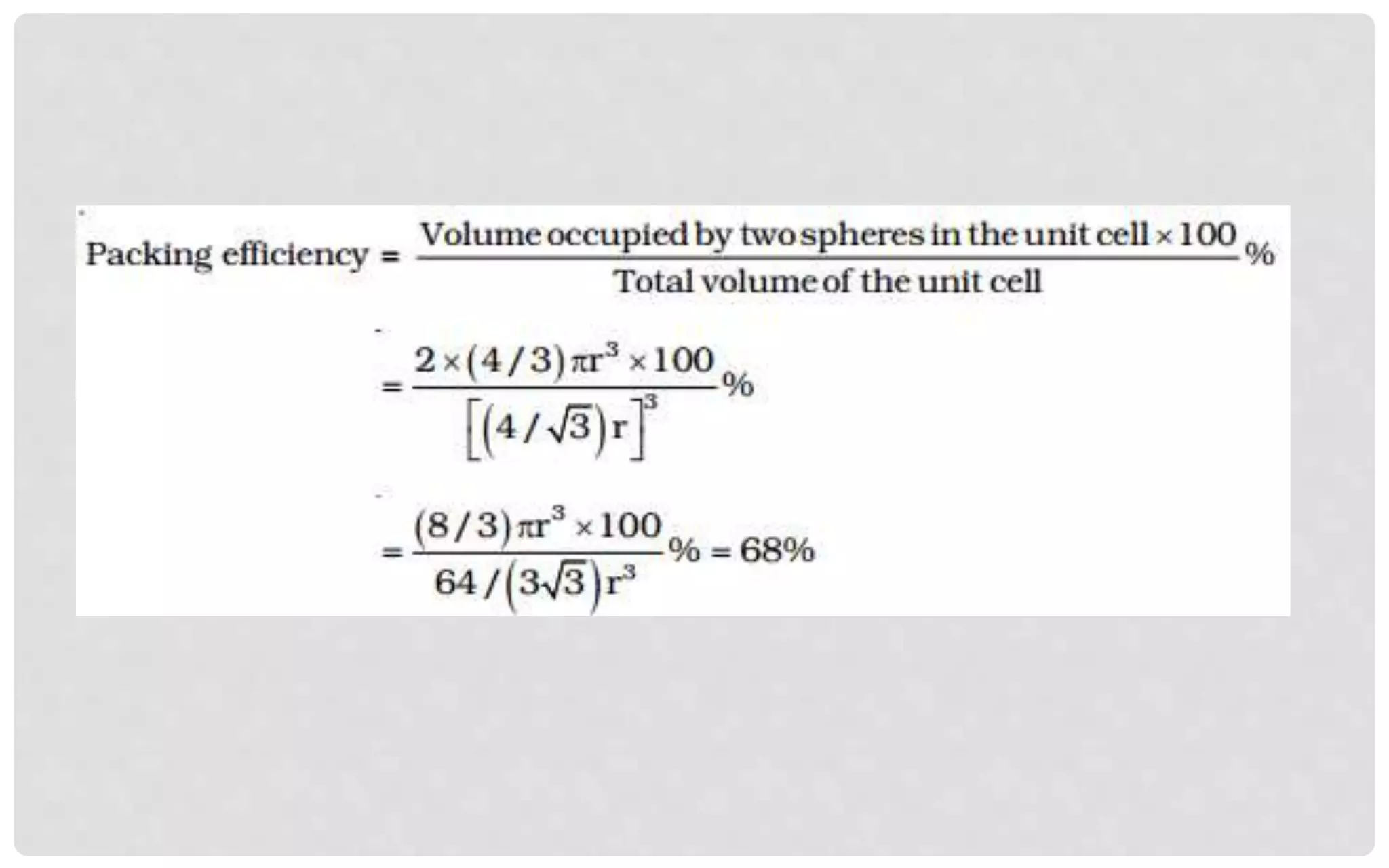
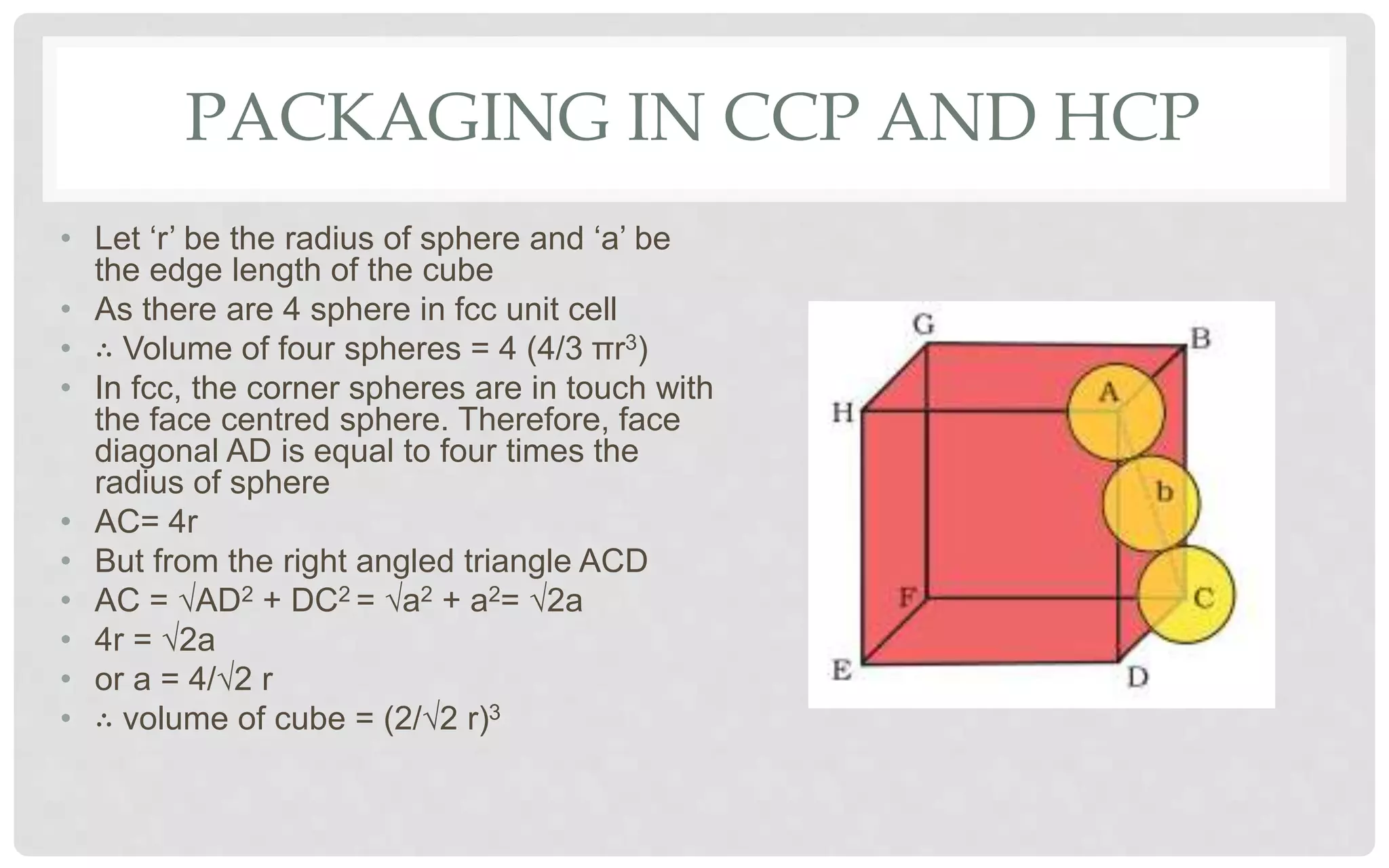
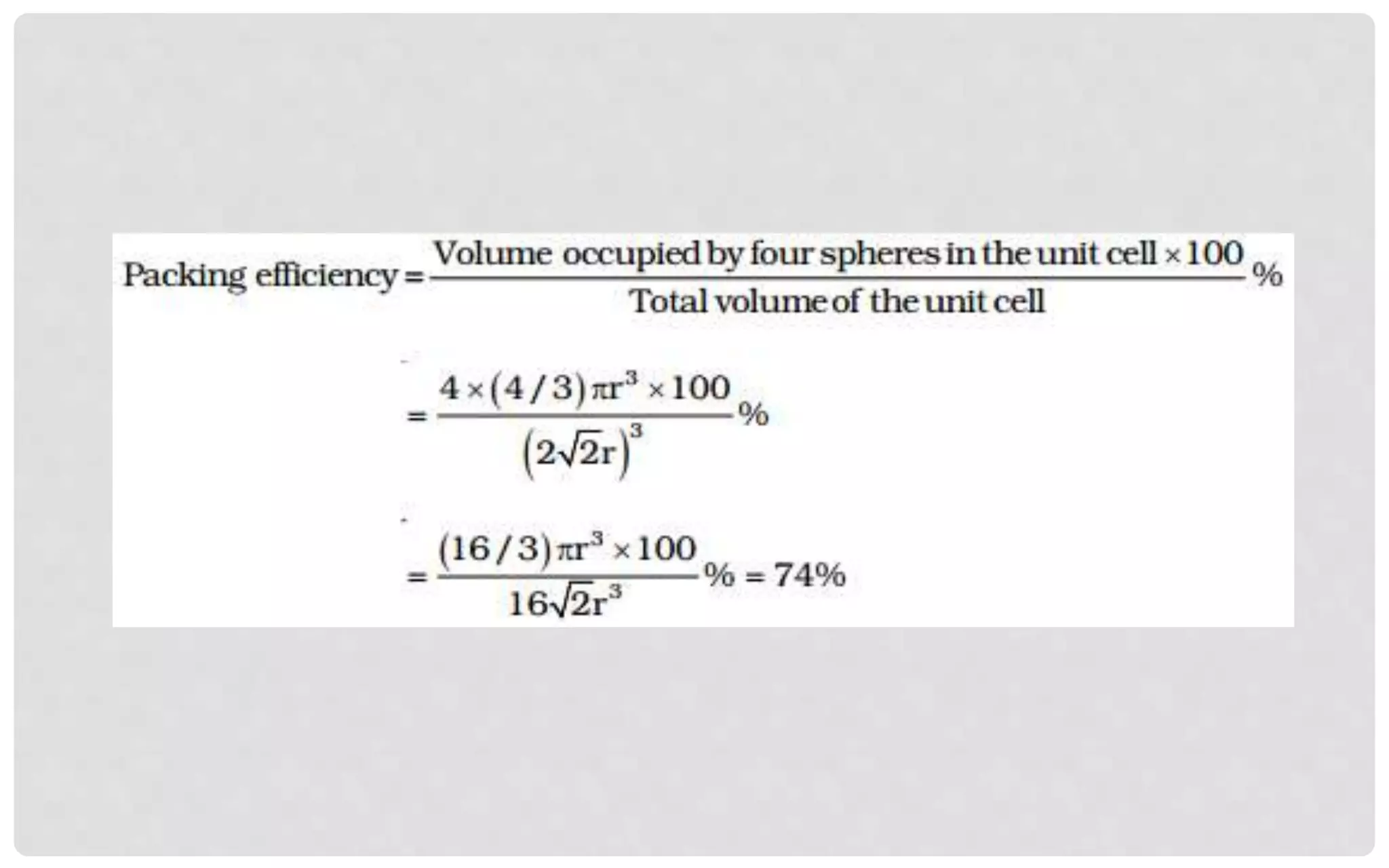
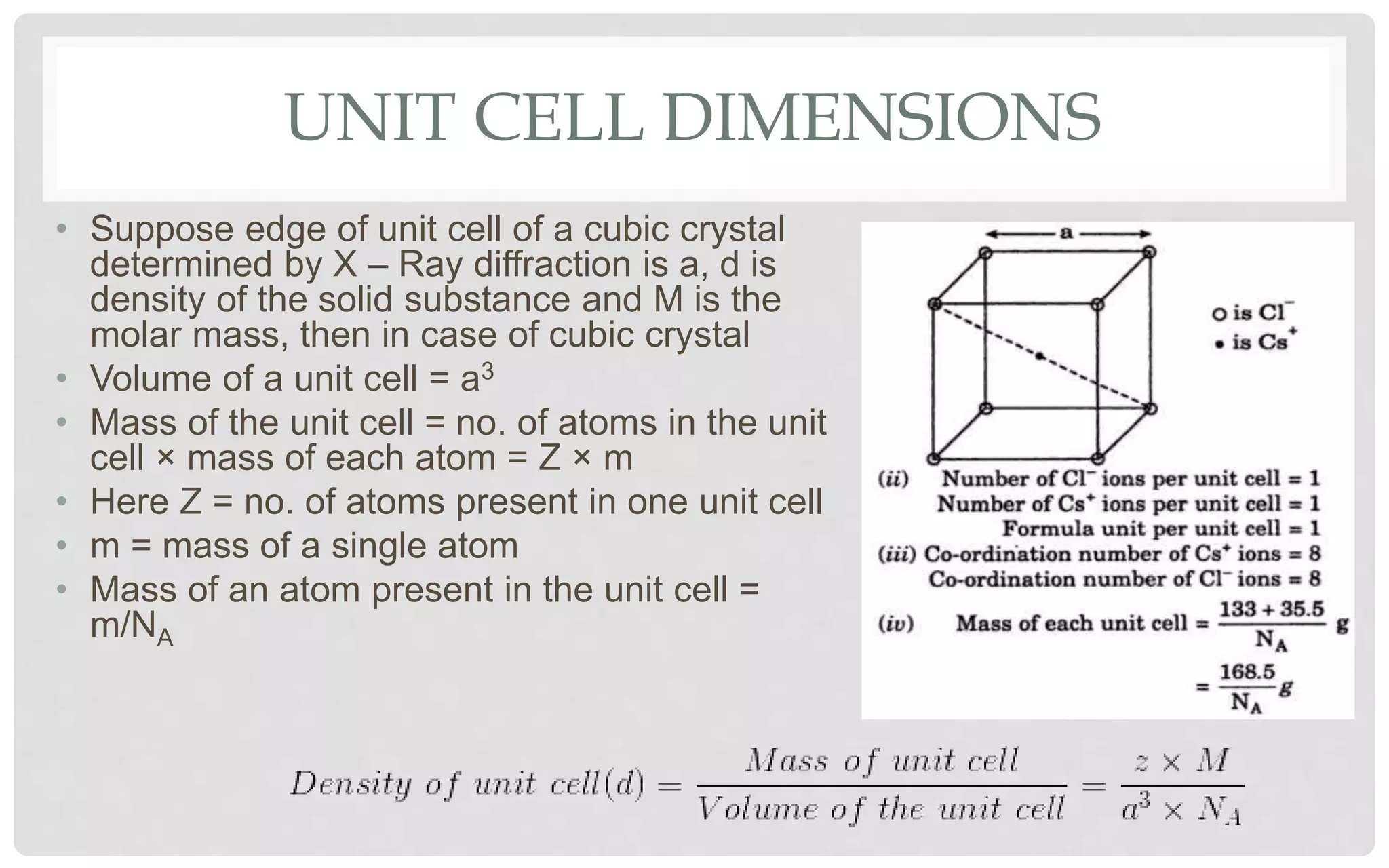
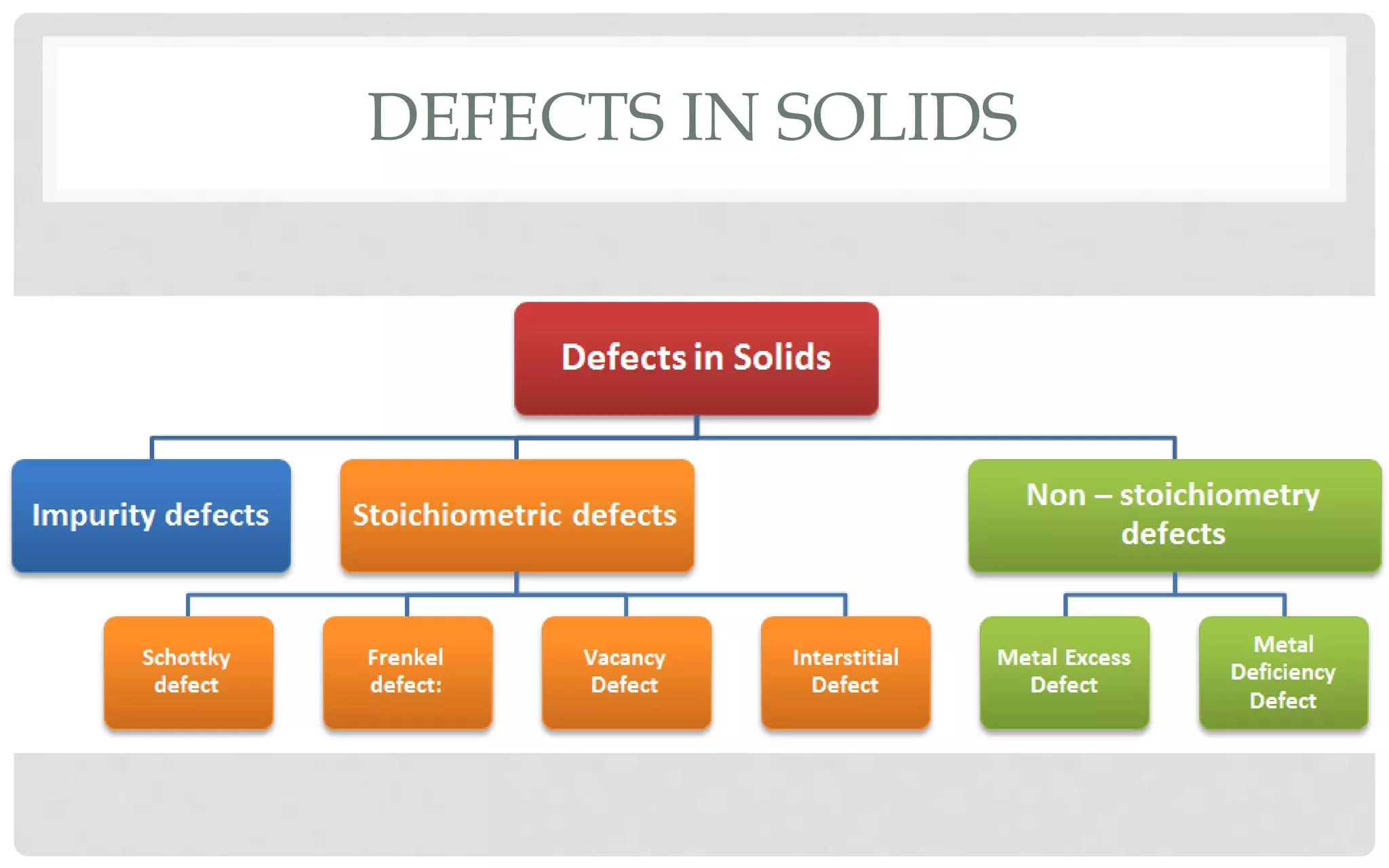
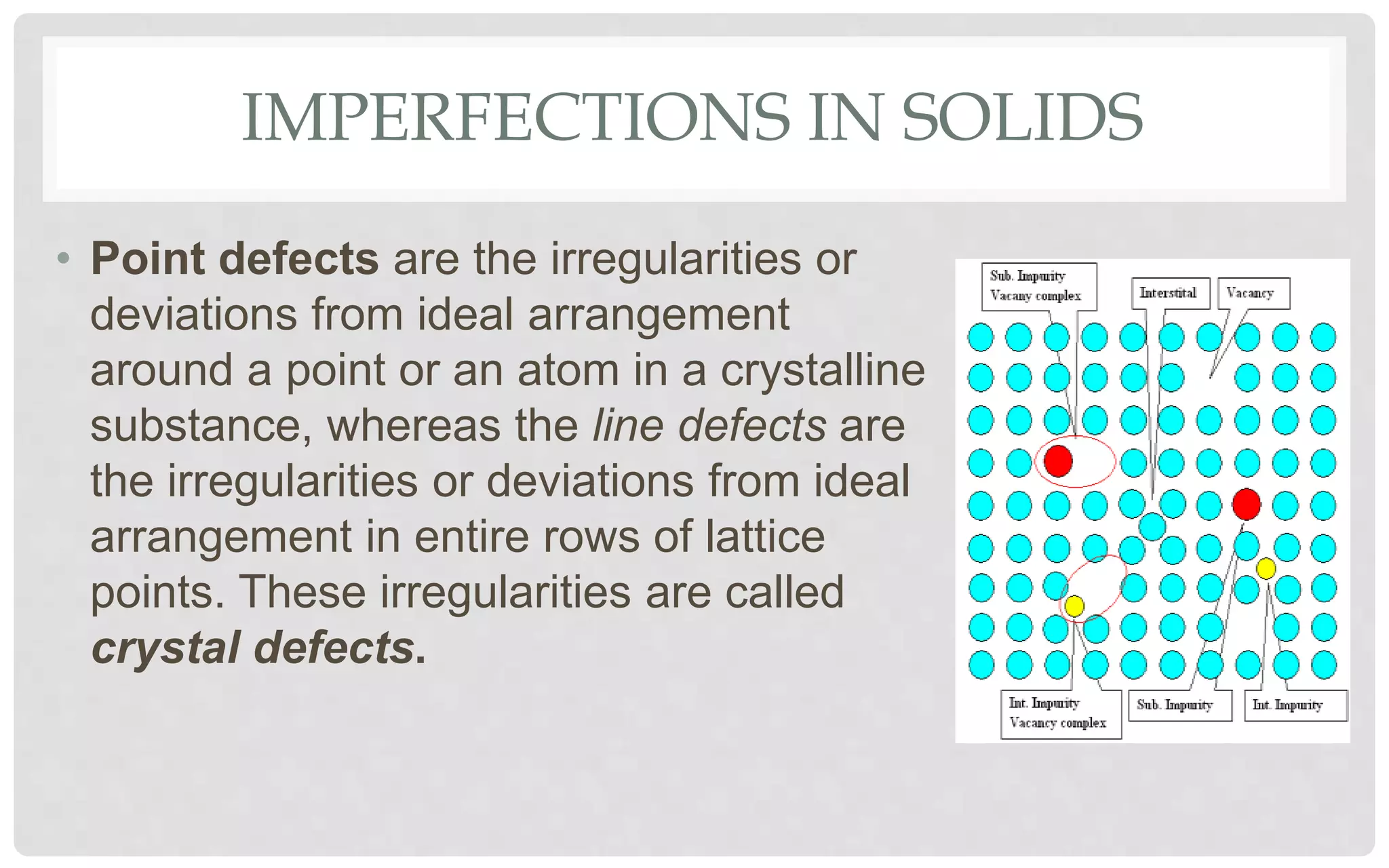
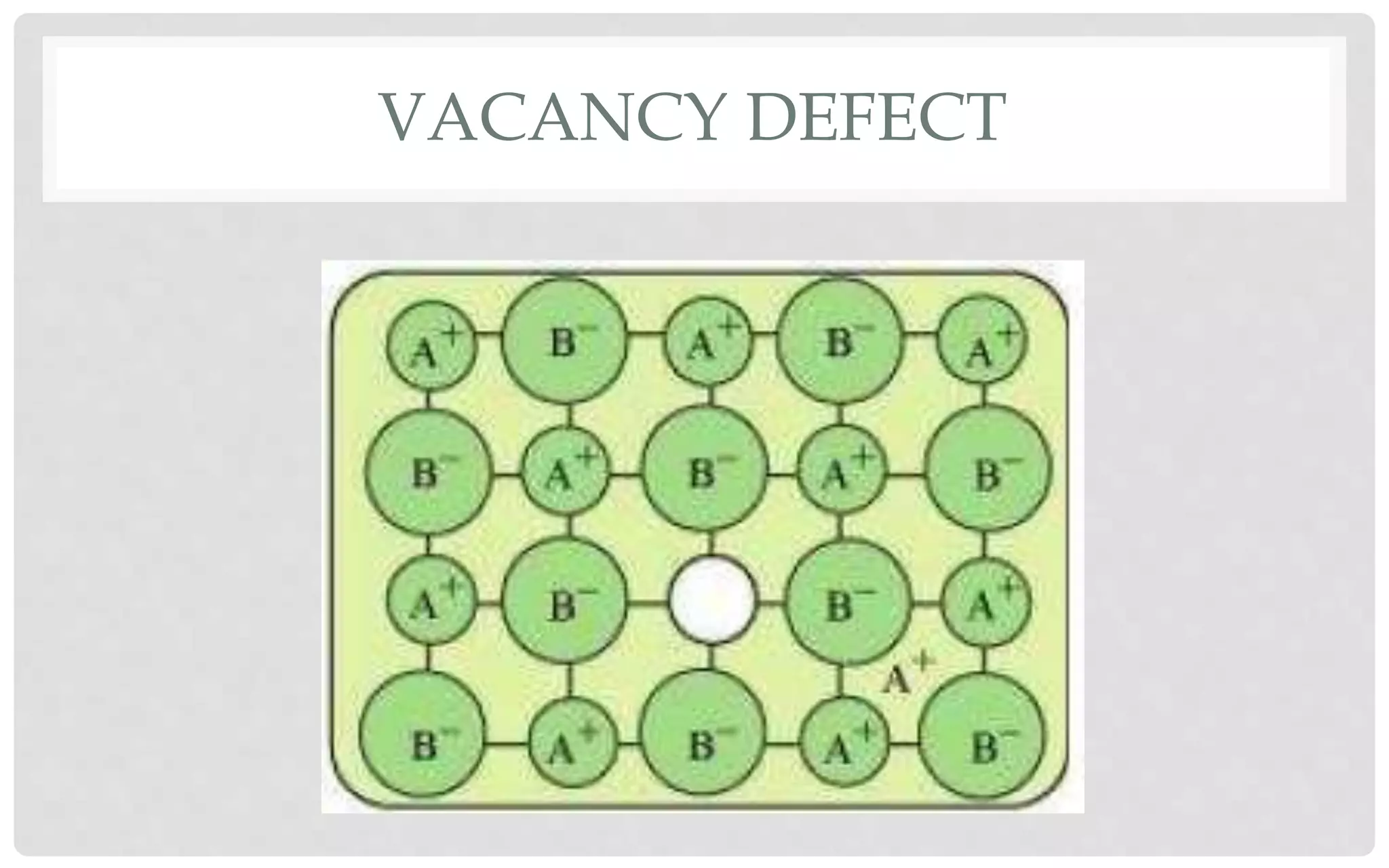
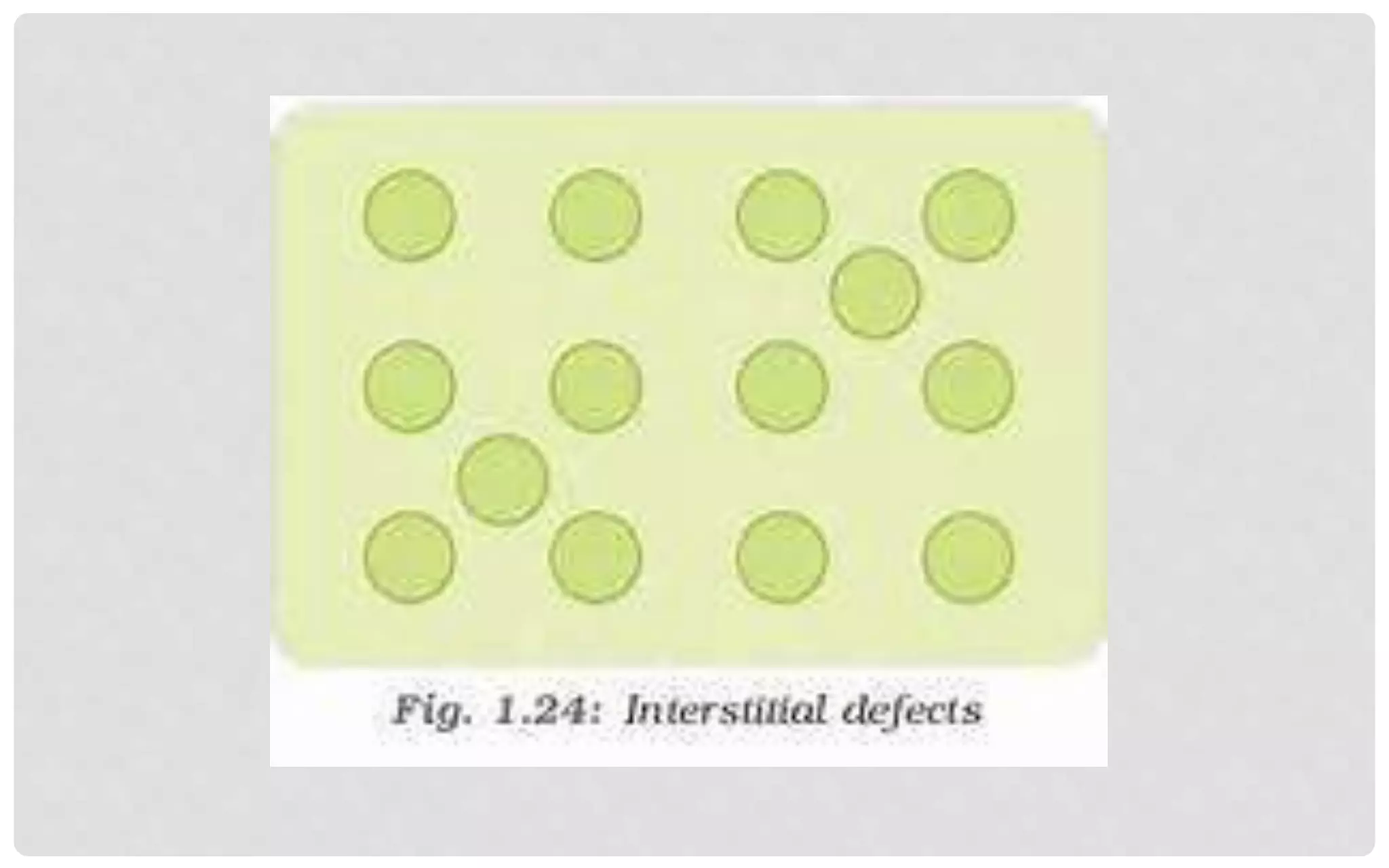
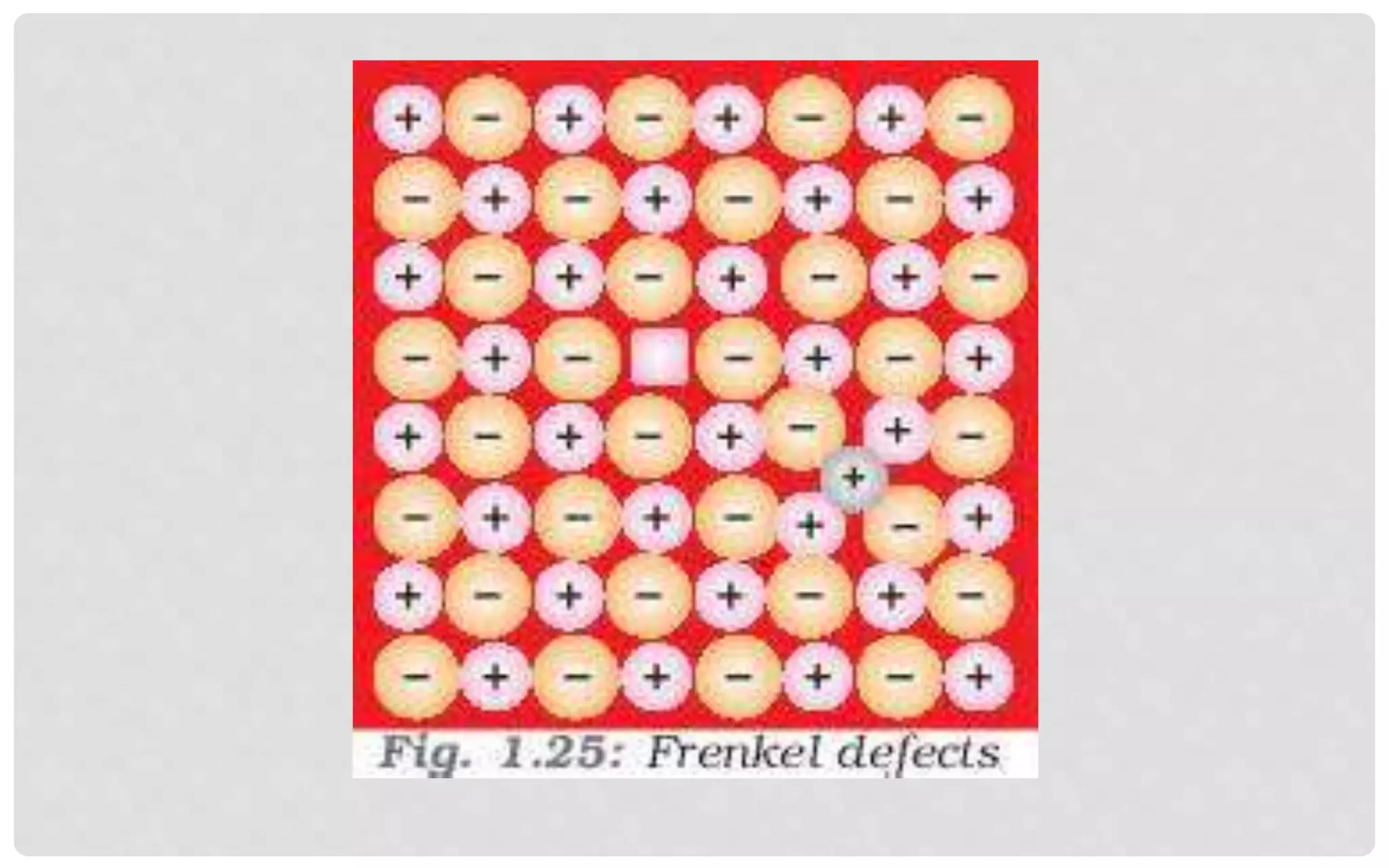
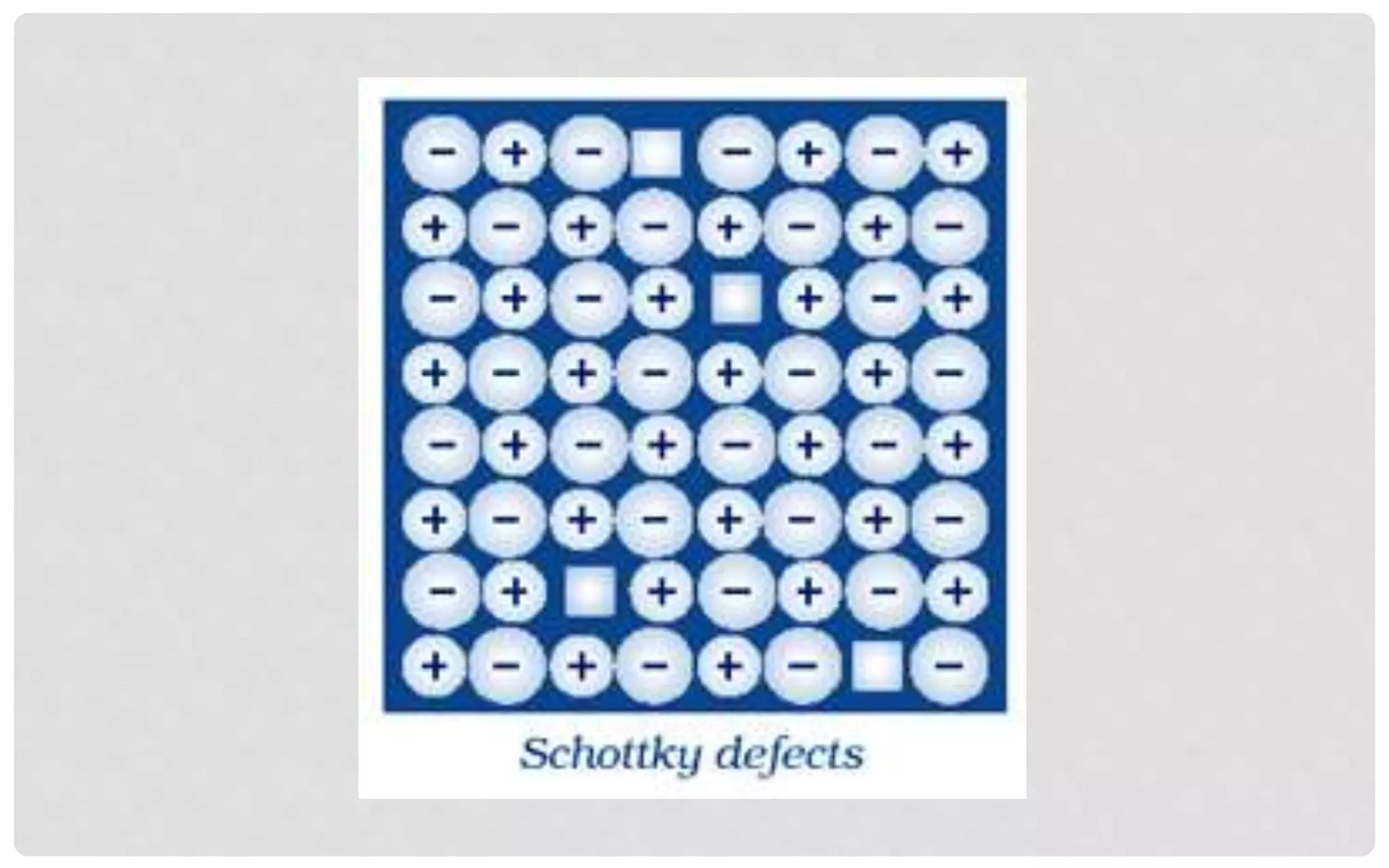
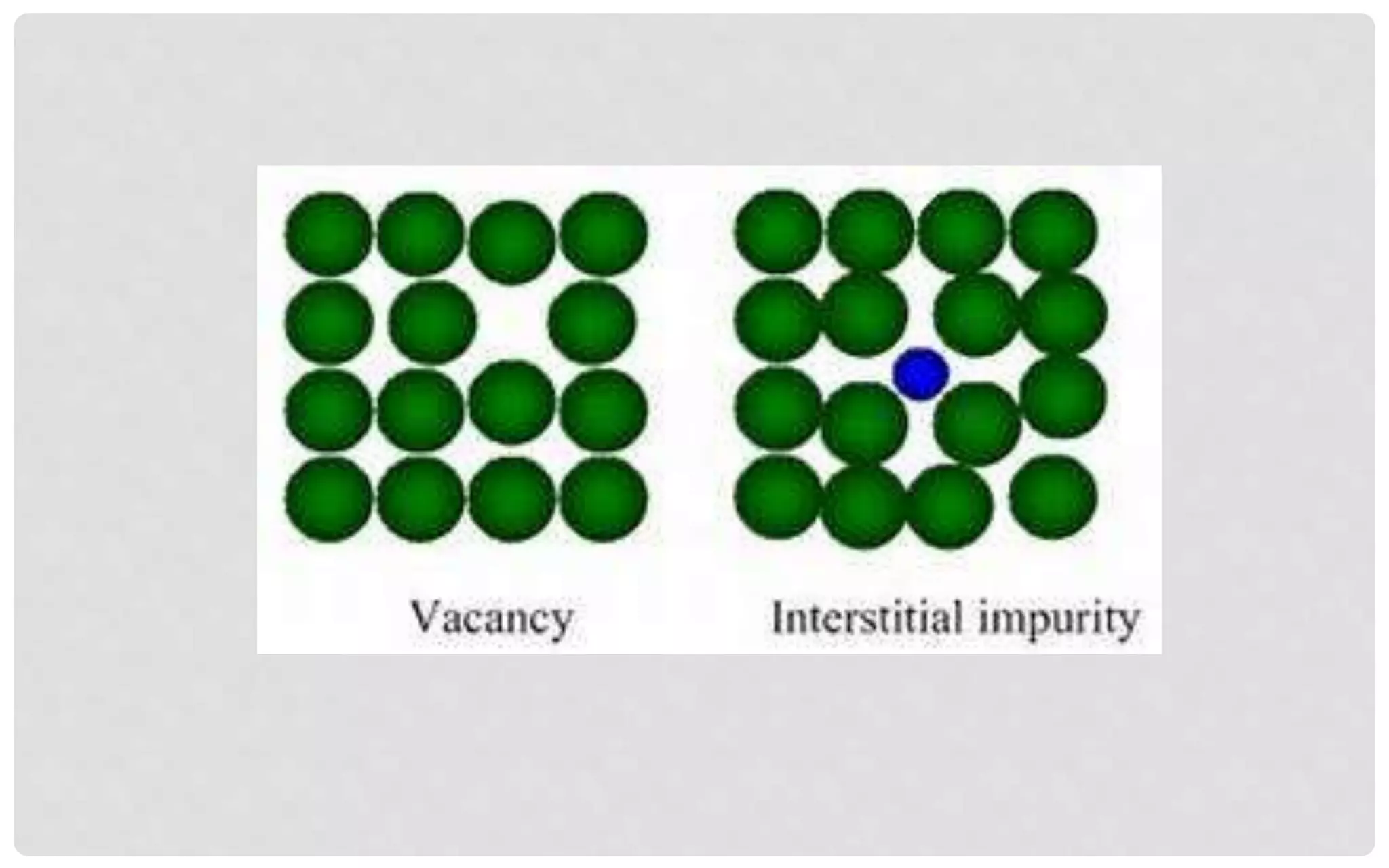
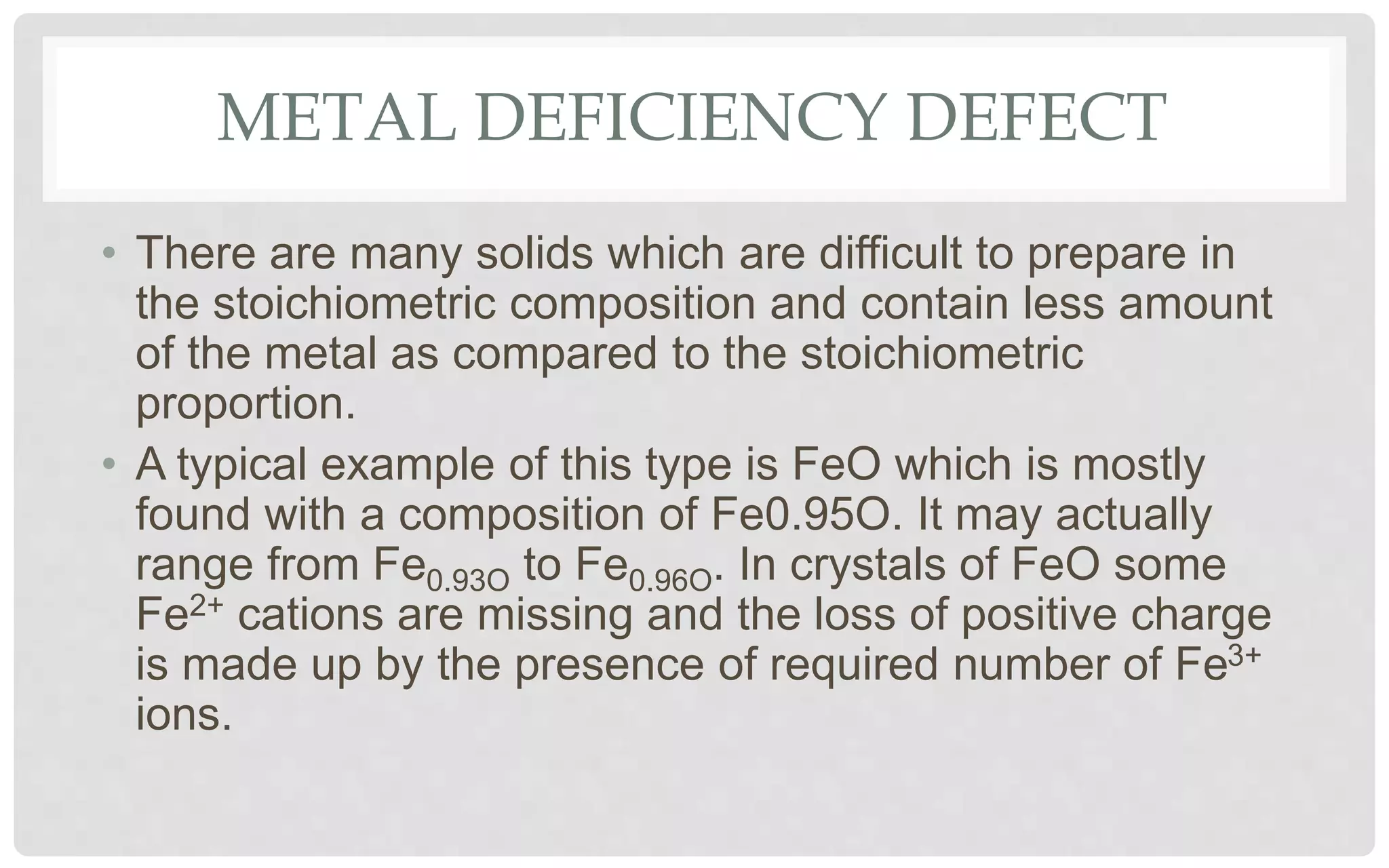
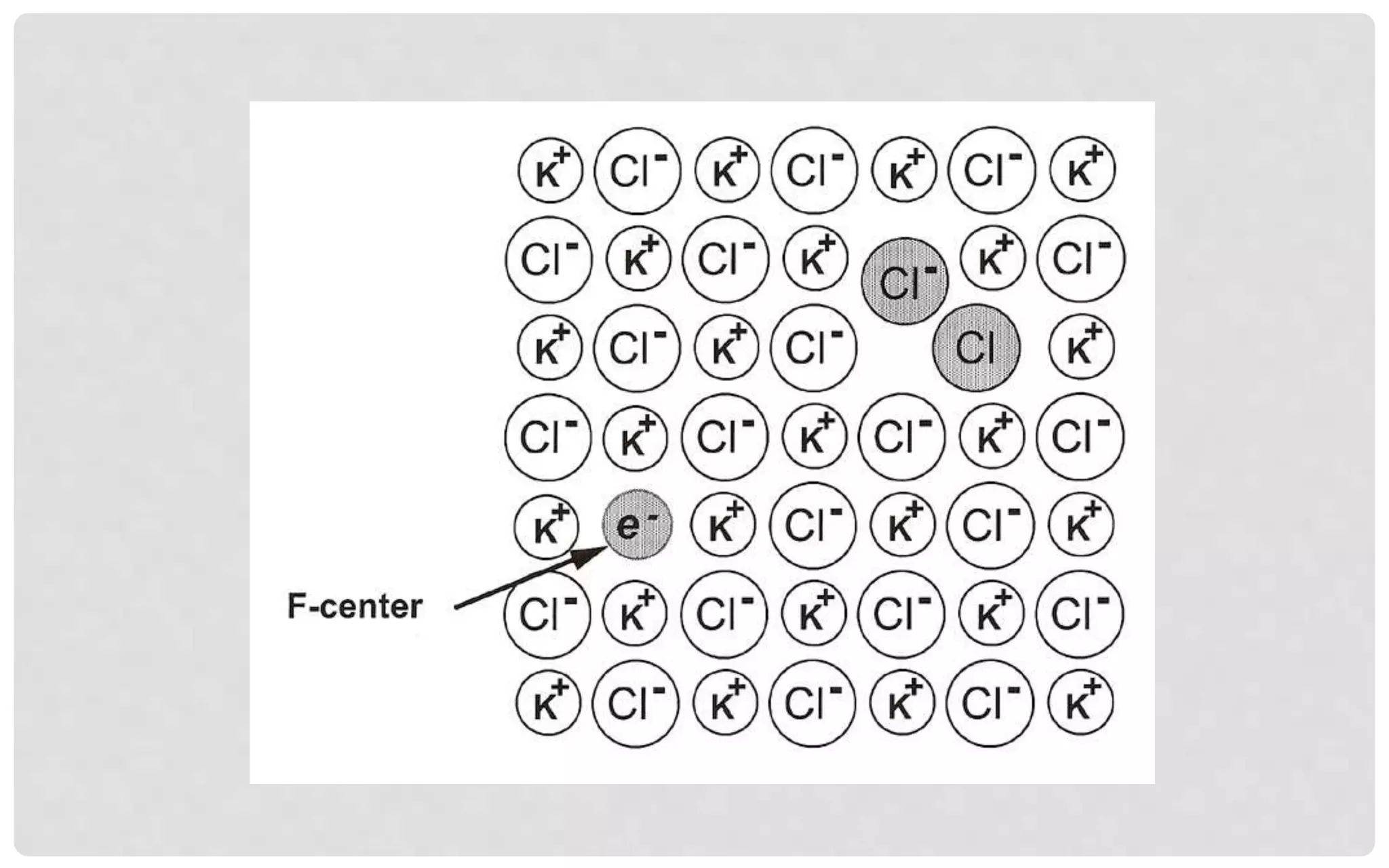
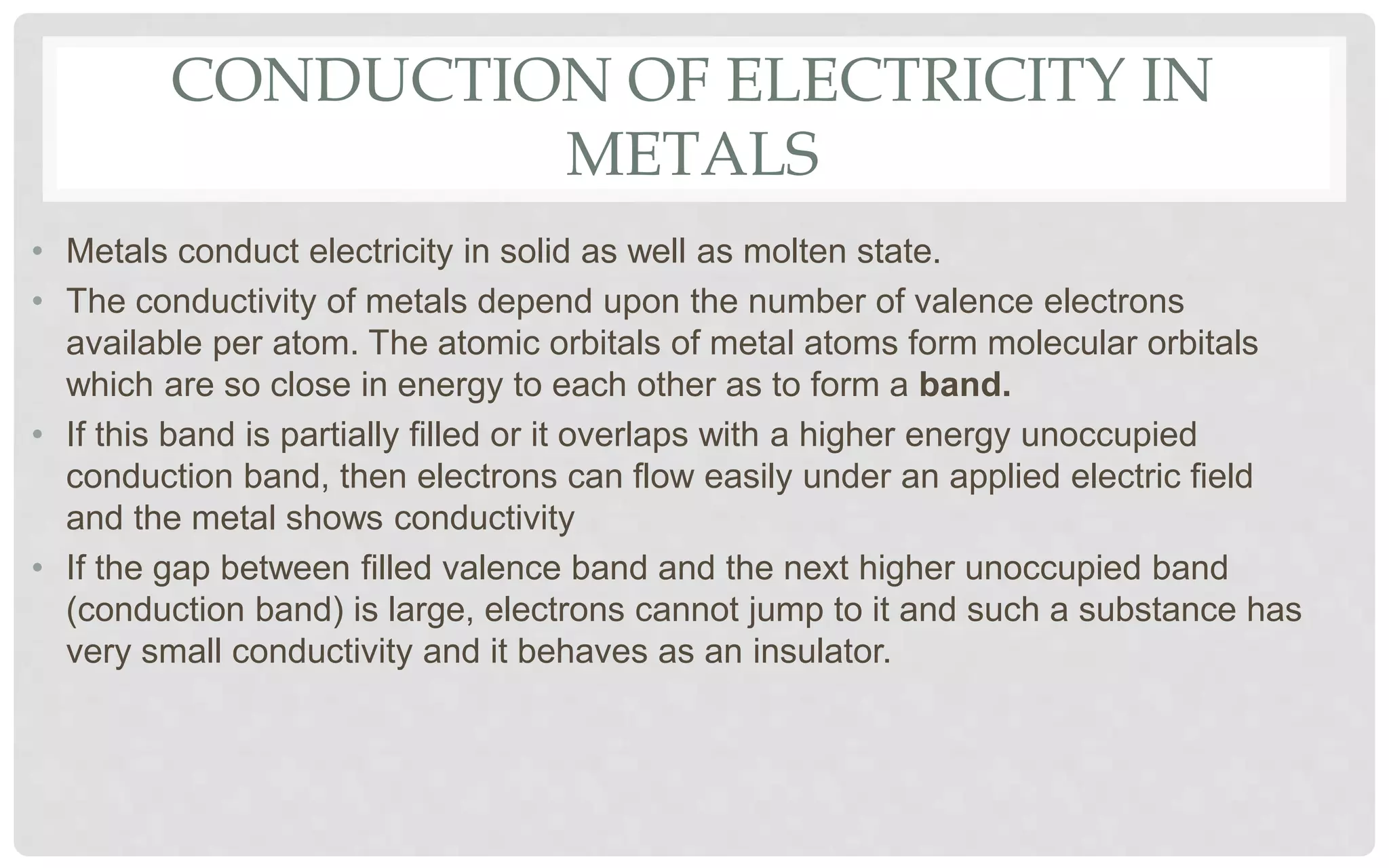
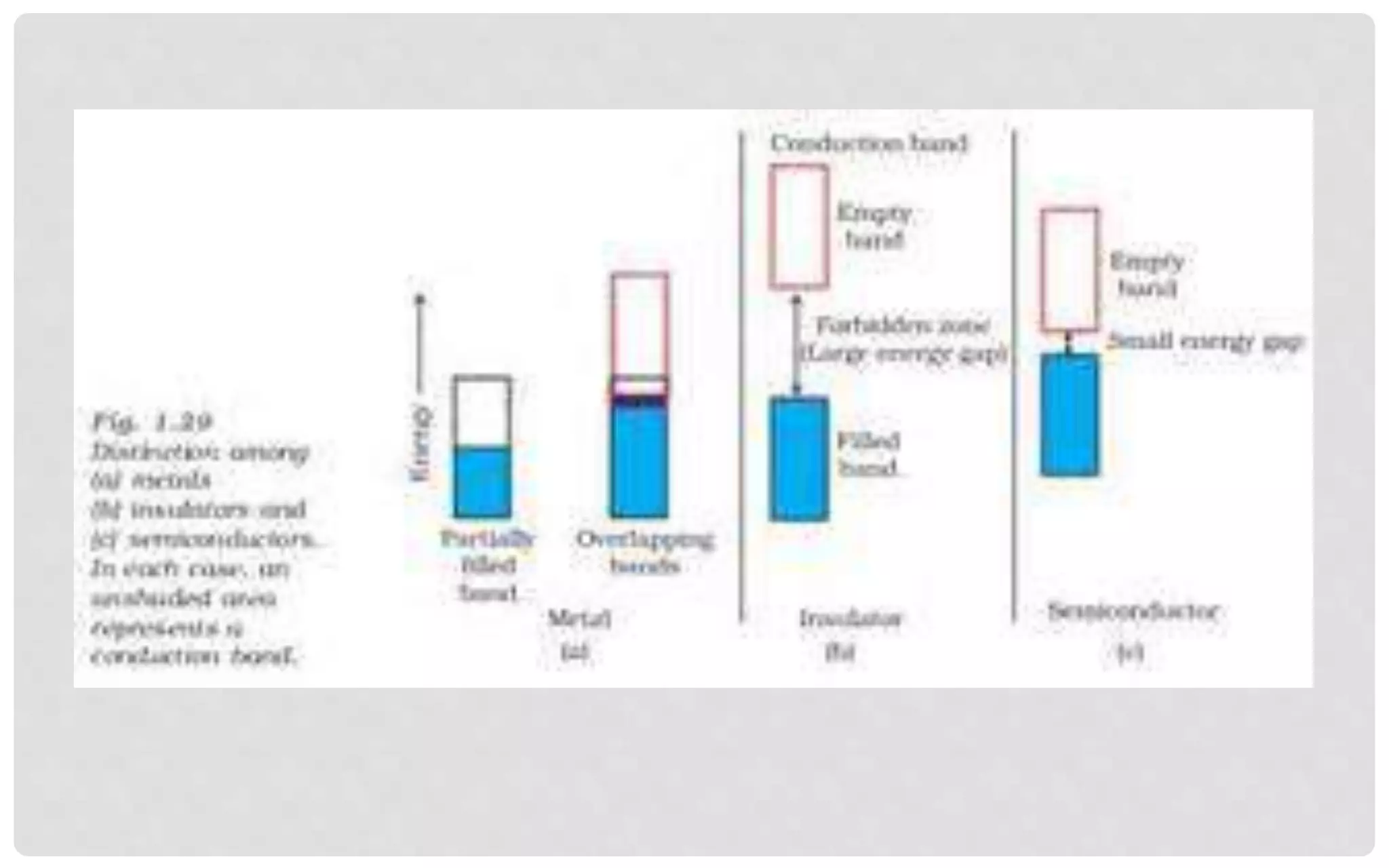
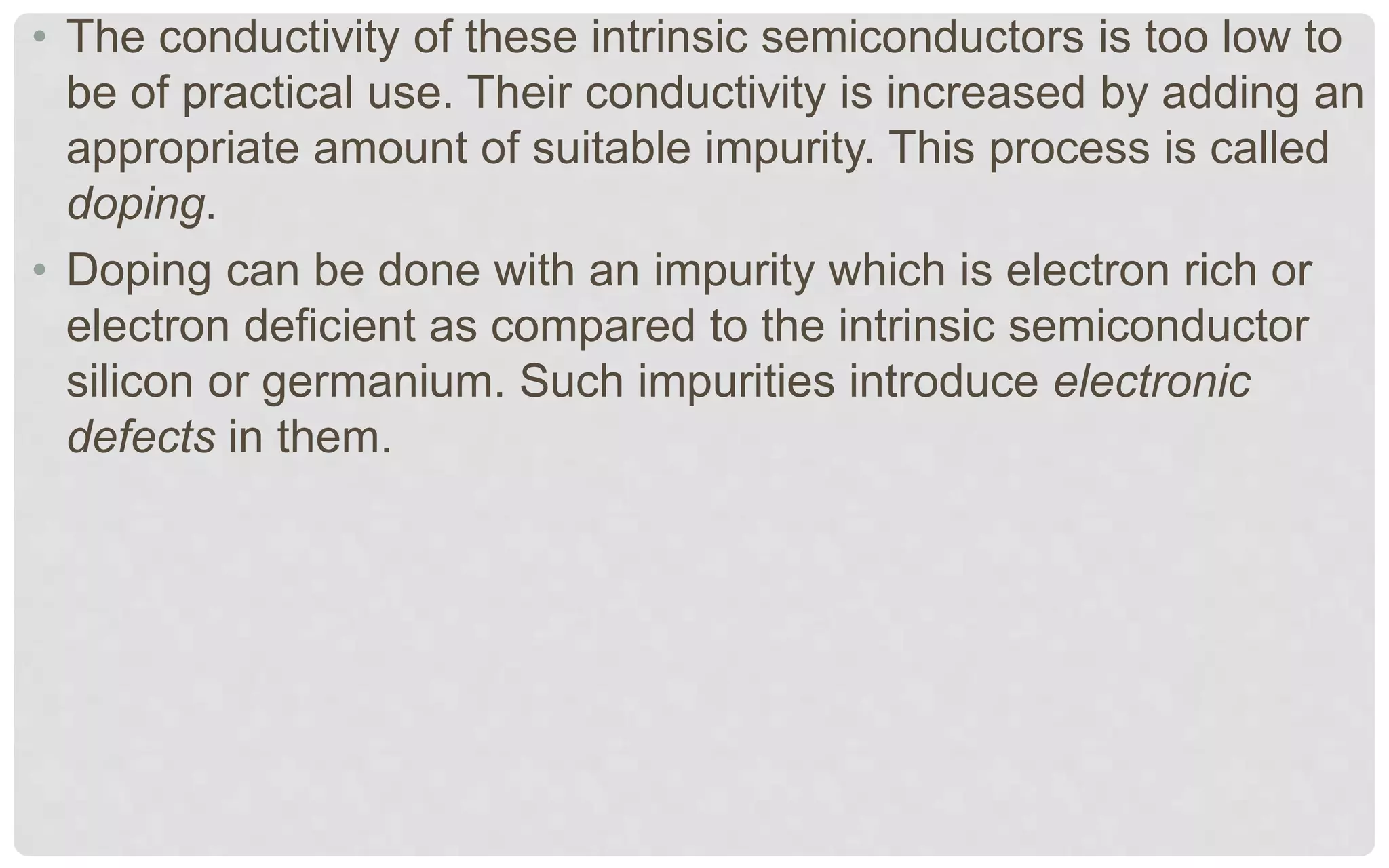
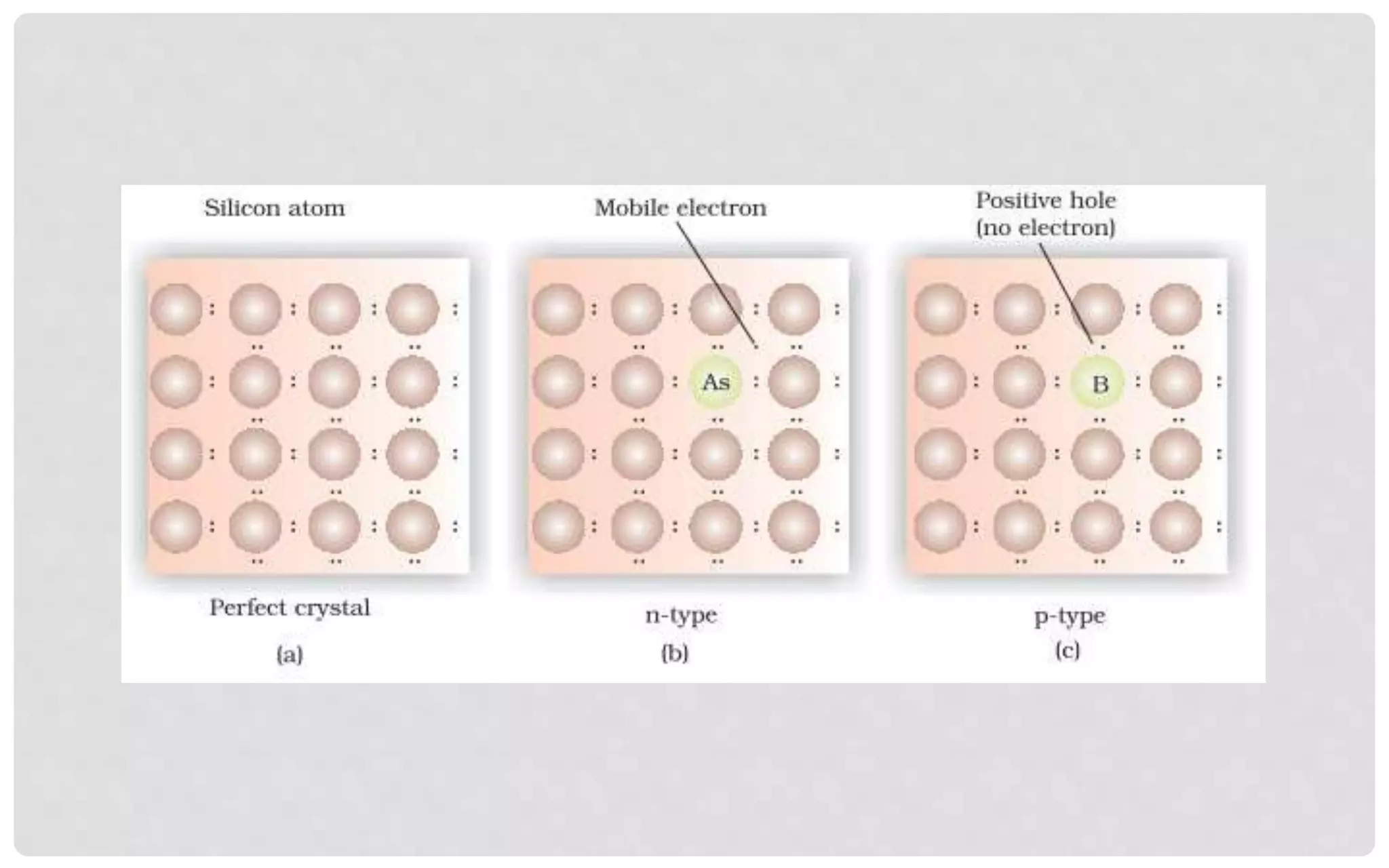
The document discusses the characteristics of solids and different types of crystalline structures. It describes that solids can be crystalline or amorphous based on the ordering of particles. Crystalline solids have long-range order and a repeating pattern, while amorphous solids only have short-range order. Crystalline solids are further classified as ionic, molecular, metallic or covalent networks based on bonding. Crystals consist of lattice structures with primitive or centered unit cells containing particles in specific arrangements. Close packing of spheres in one, two or three dimensions results in different crystal structures like simple cubic, body centered cubic or hexagonal close packed.