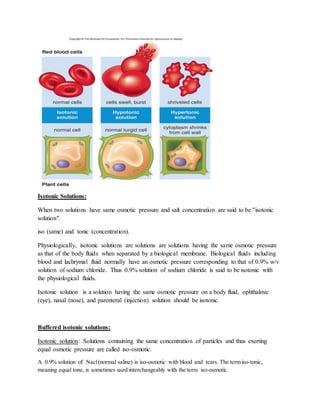
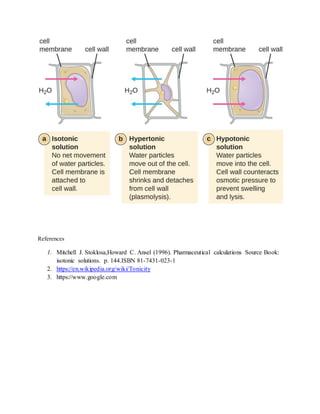
Isotonic solutions have the same osmotic pressure as body fluids like blood. Osmotic pressure depends on the number of particles in solution, including ions from electrolyte dissociation. Solutions with lower osmotic pressure than body fluids are hypotonic, while those with higher pressure are hypertonic. Physiological fluids have an osmotic pressure matching a 0.9% sodium chloride solution, which is therefore considered isotonic. Common isotonic solutions include saline and ringer's solution.