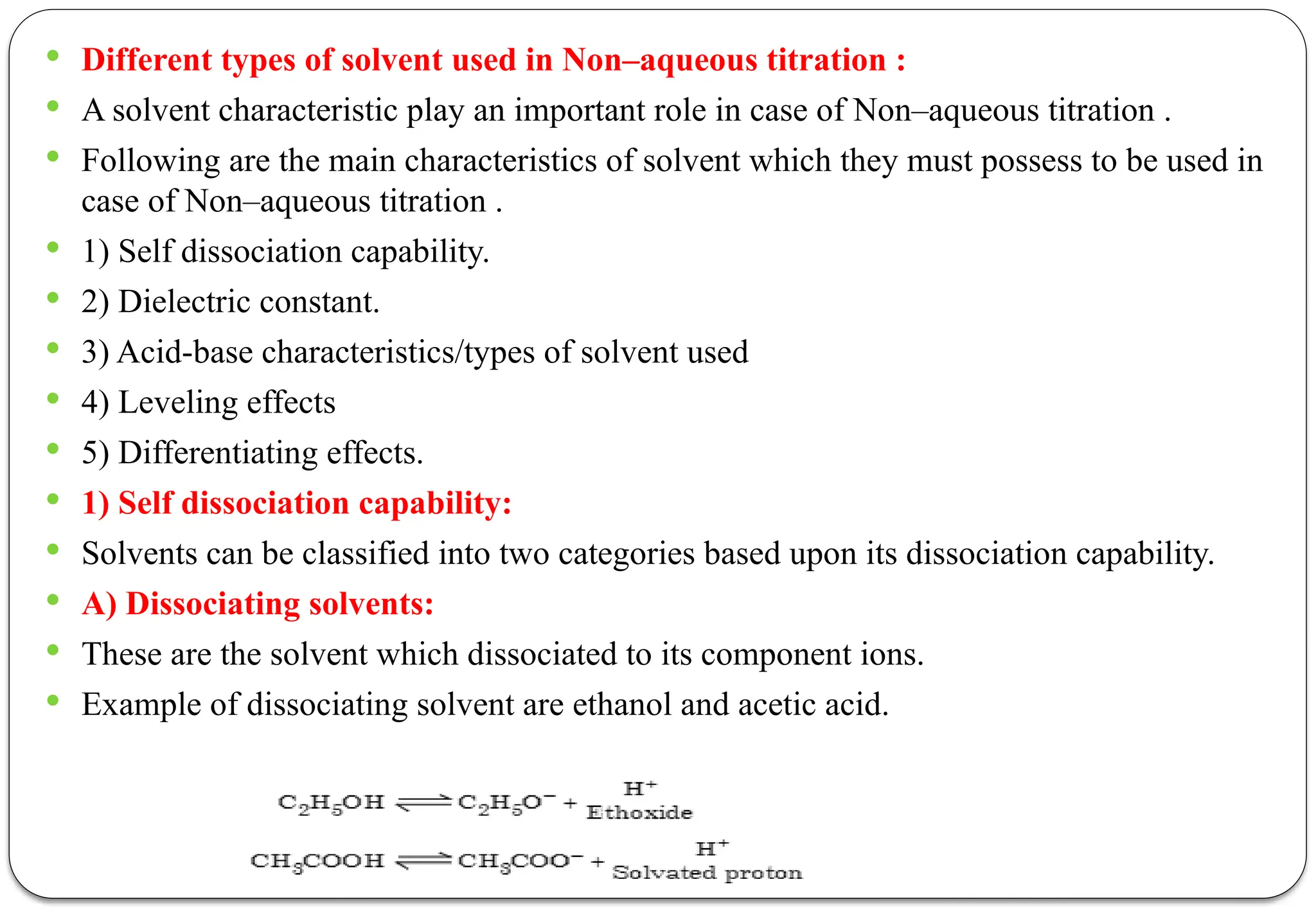
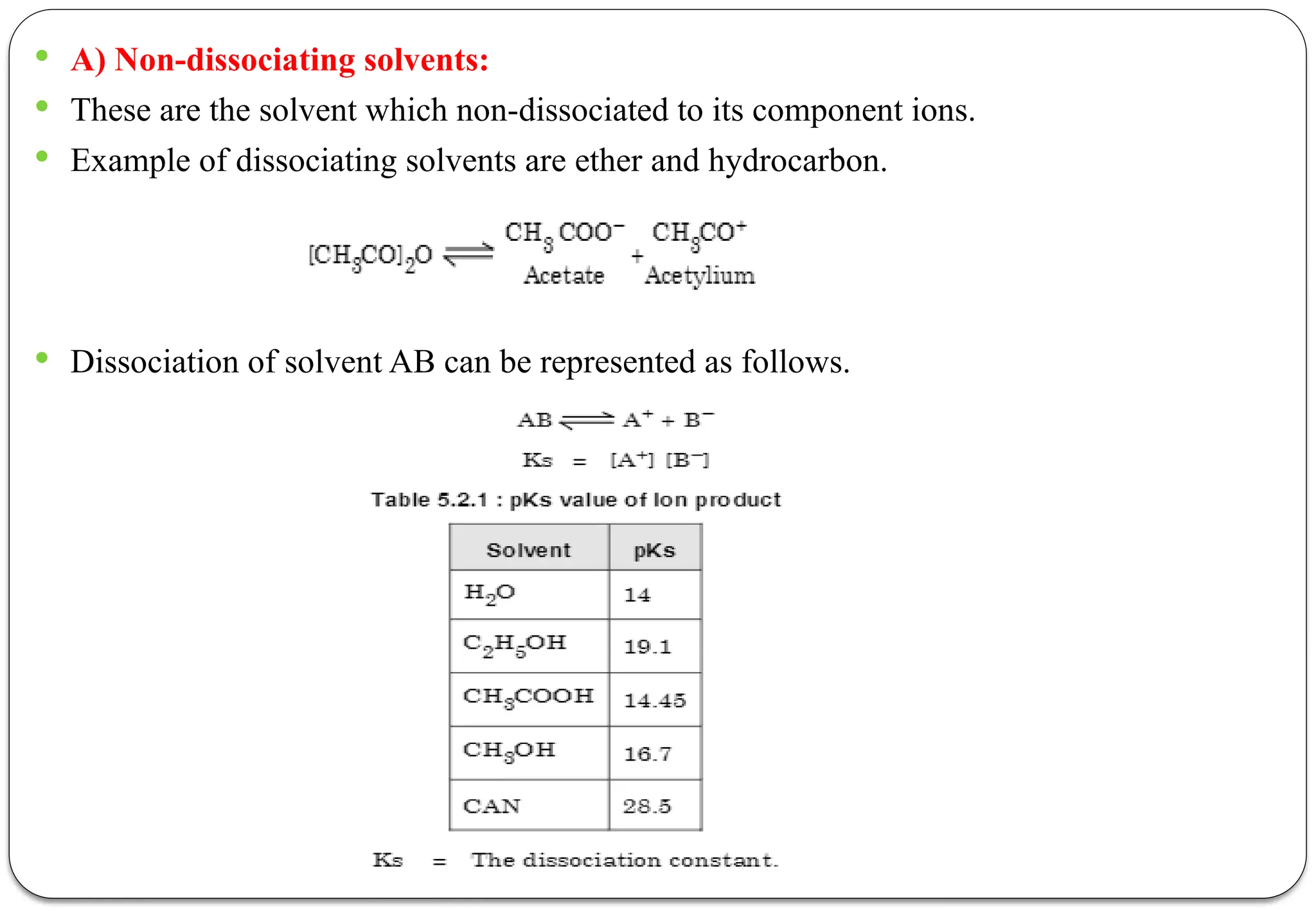
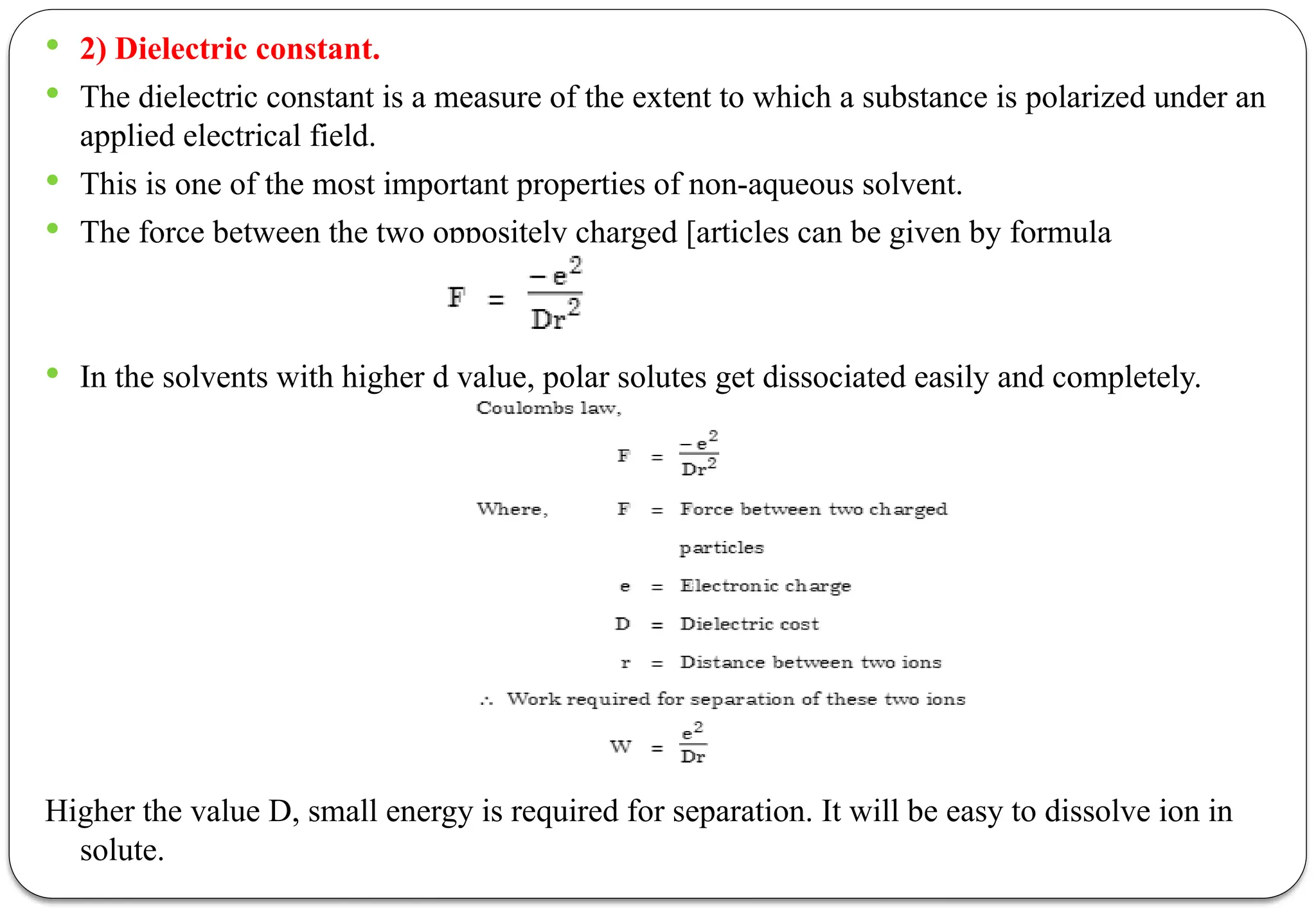
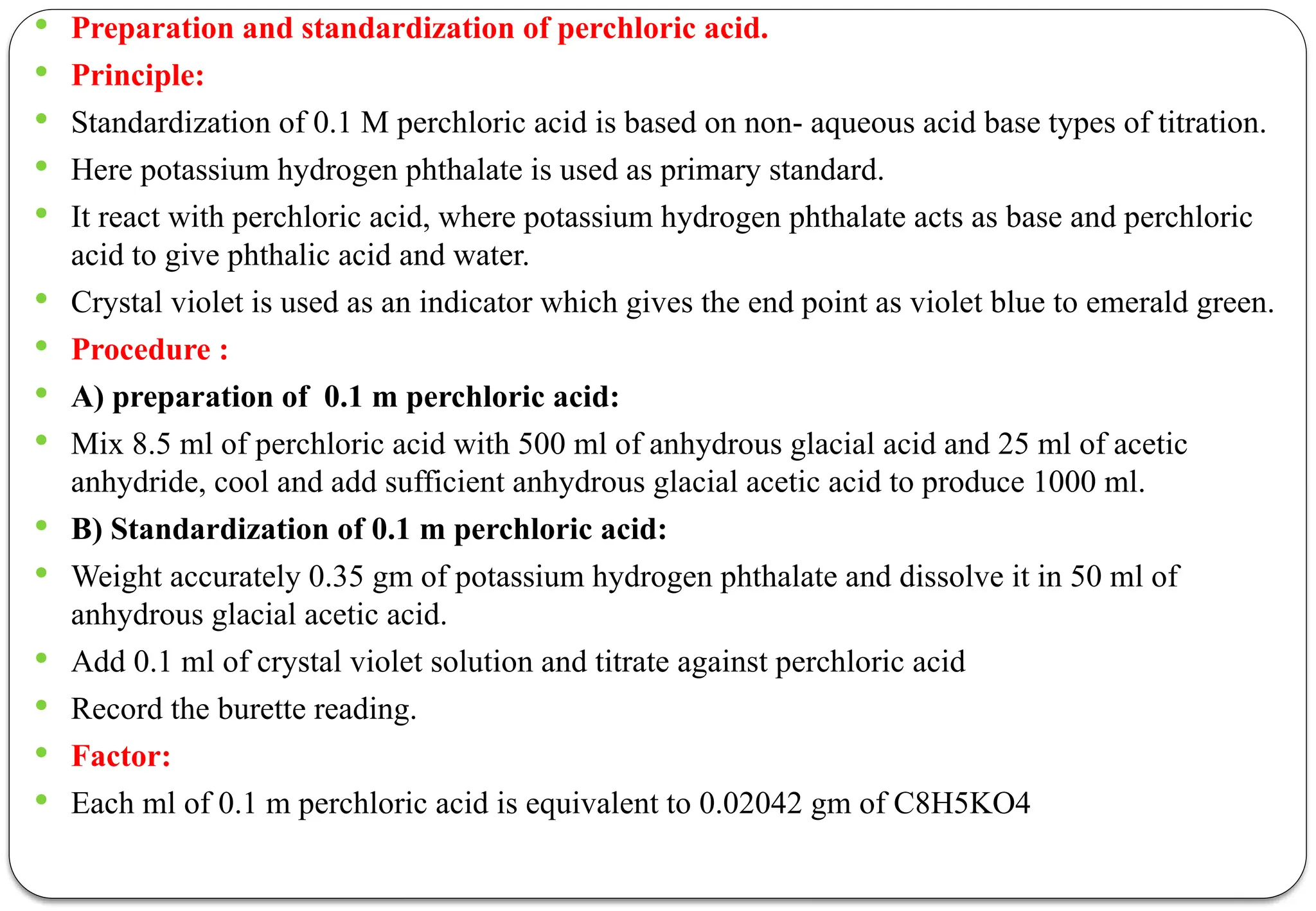
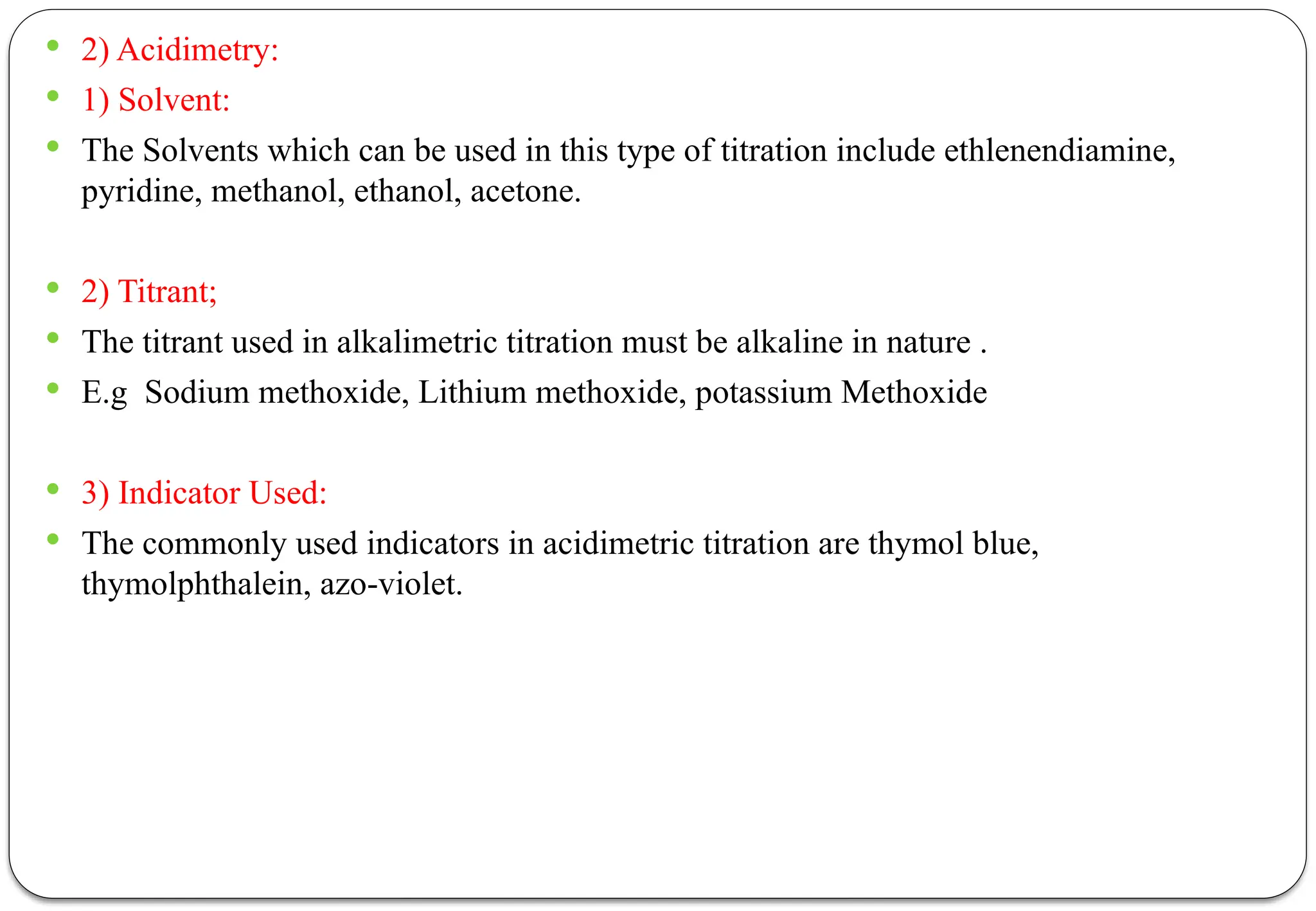
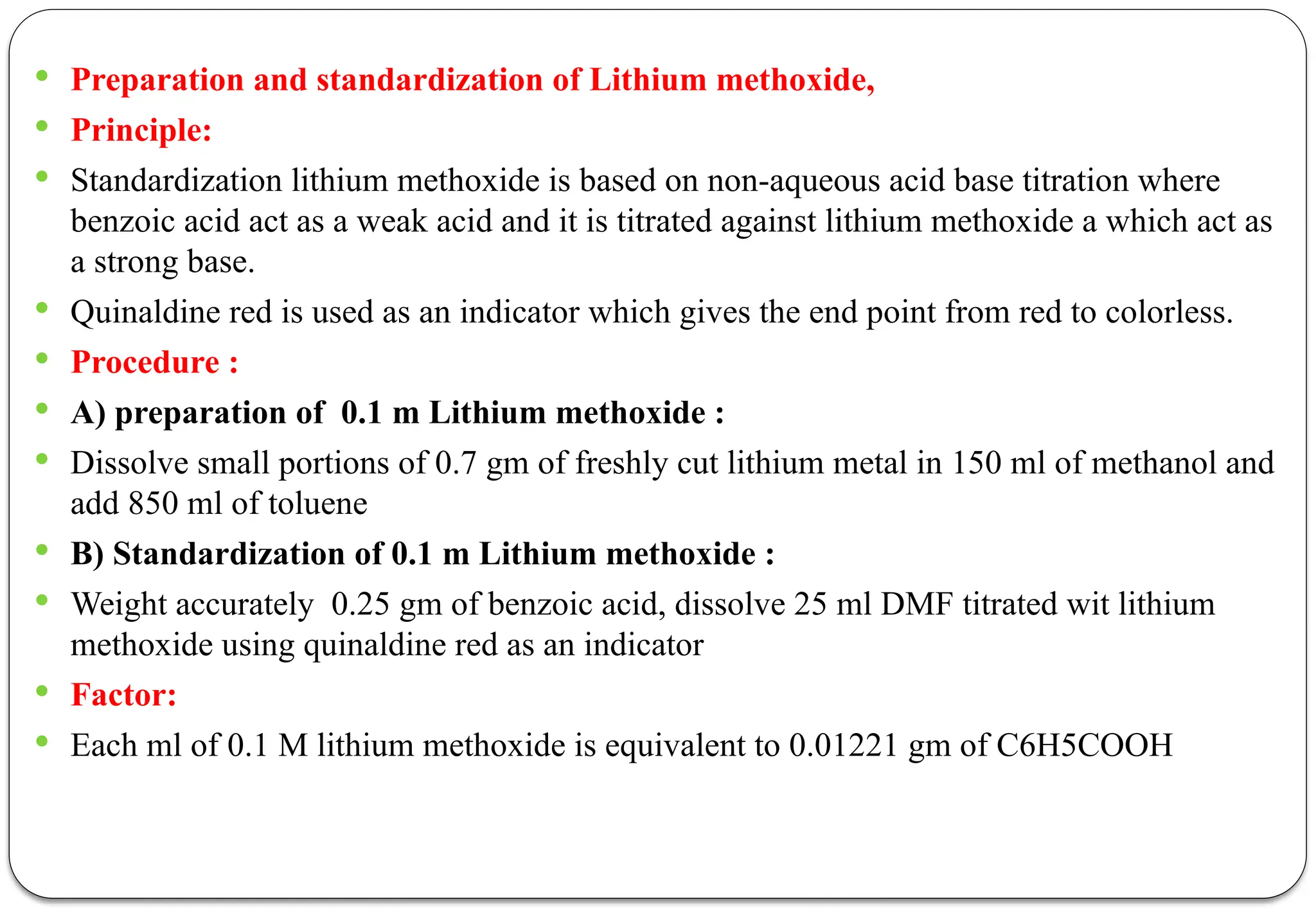
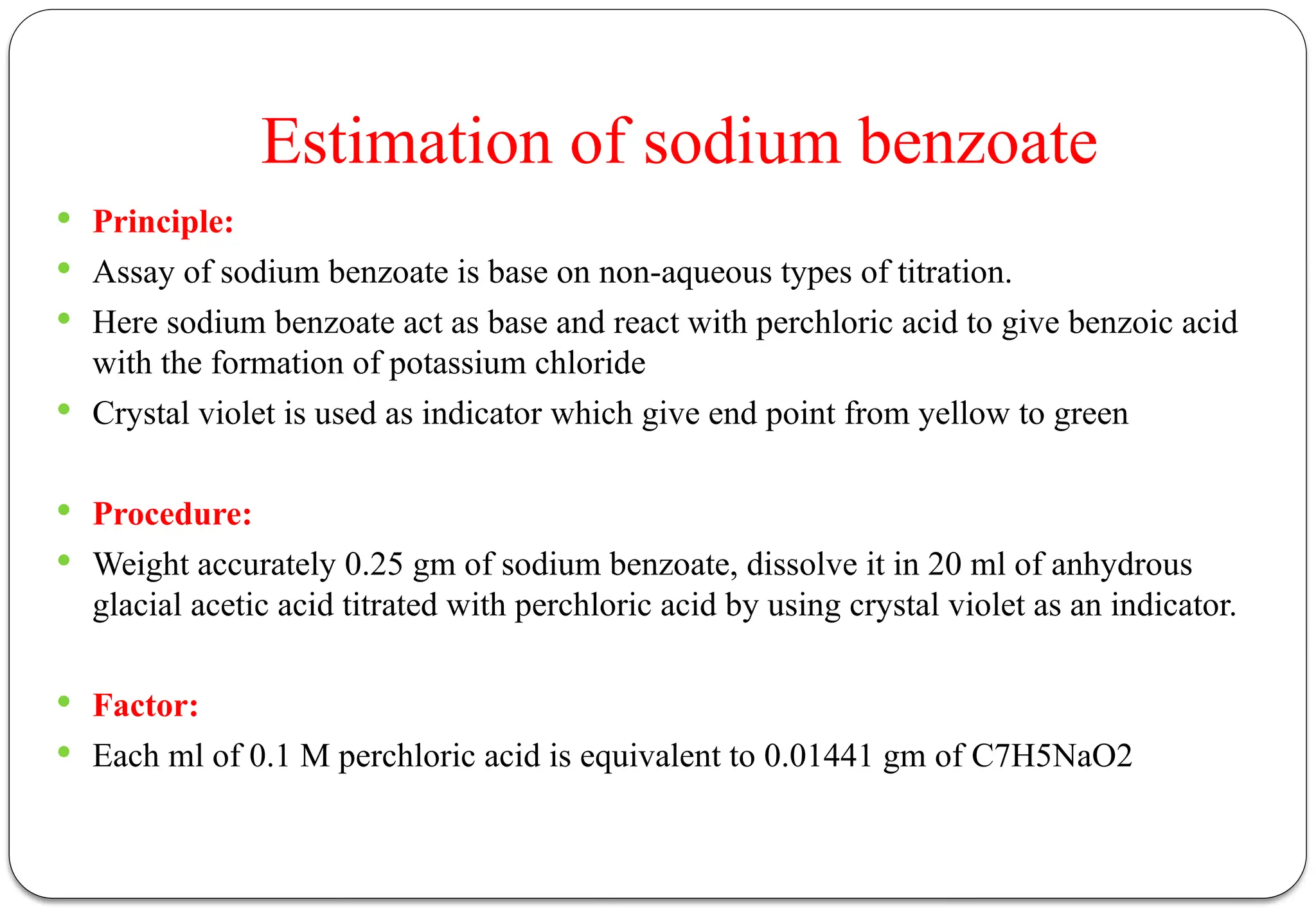
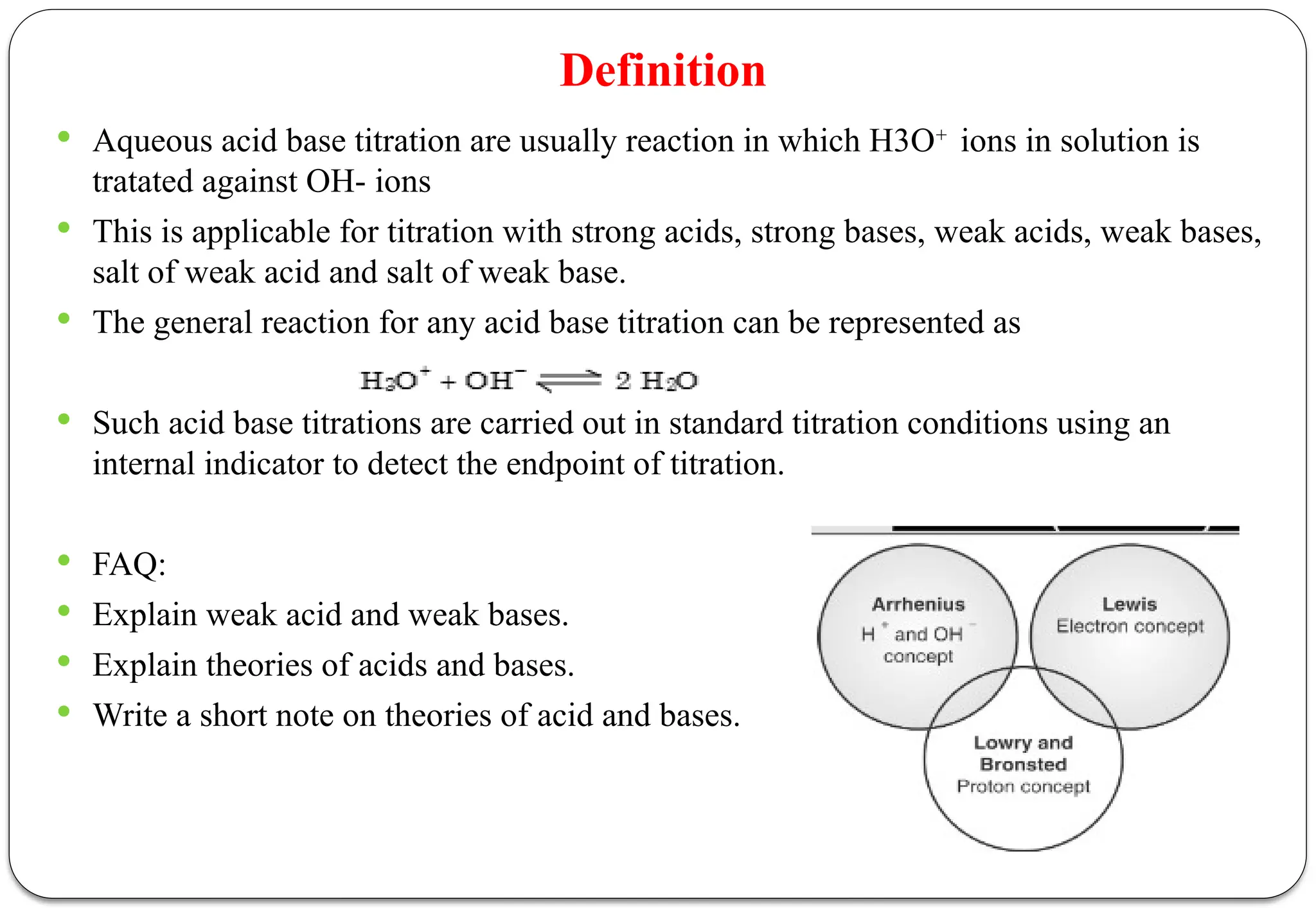
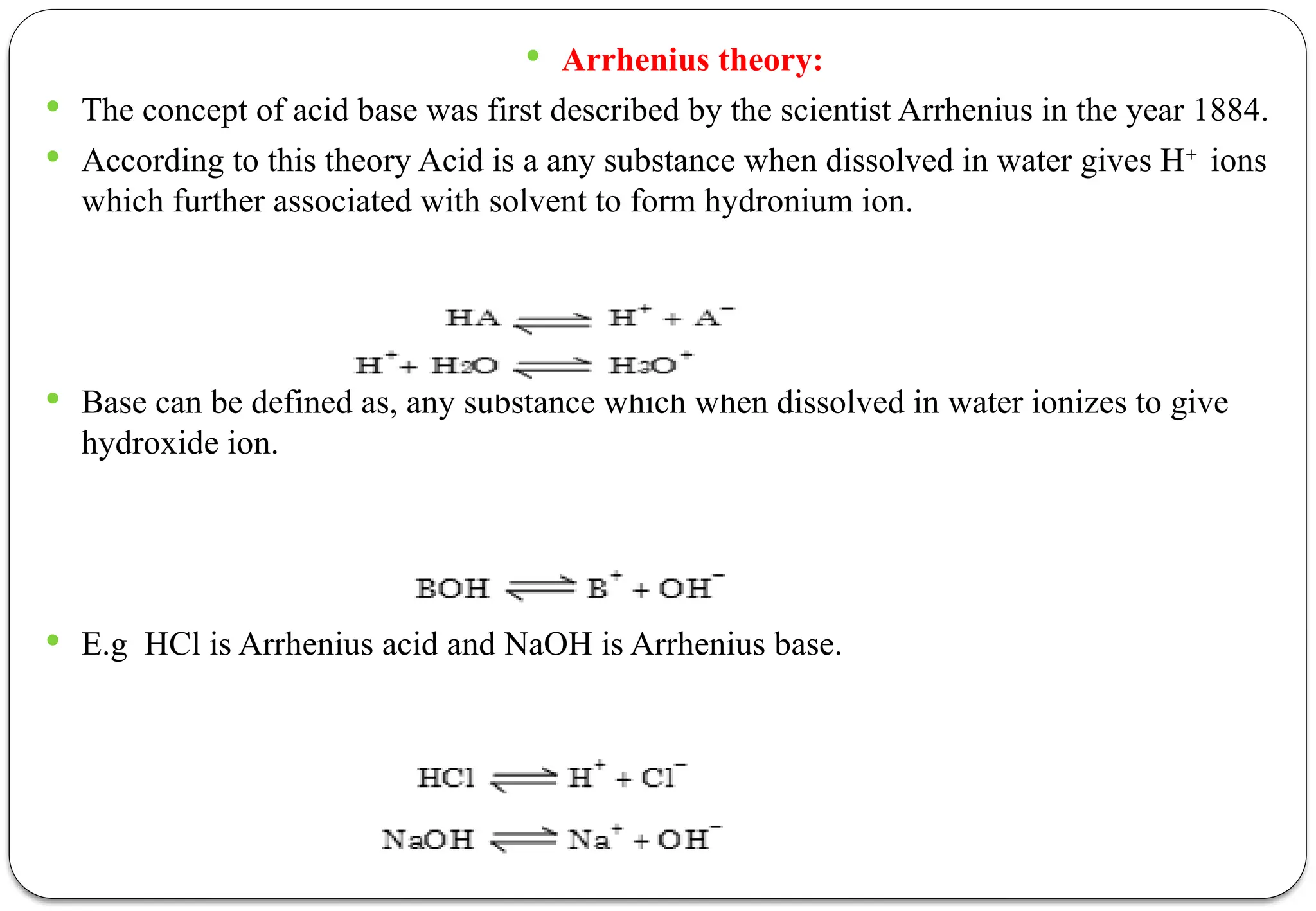
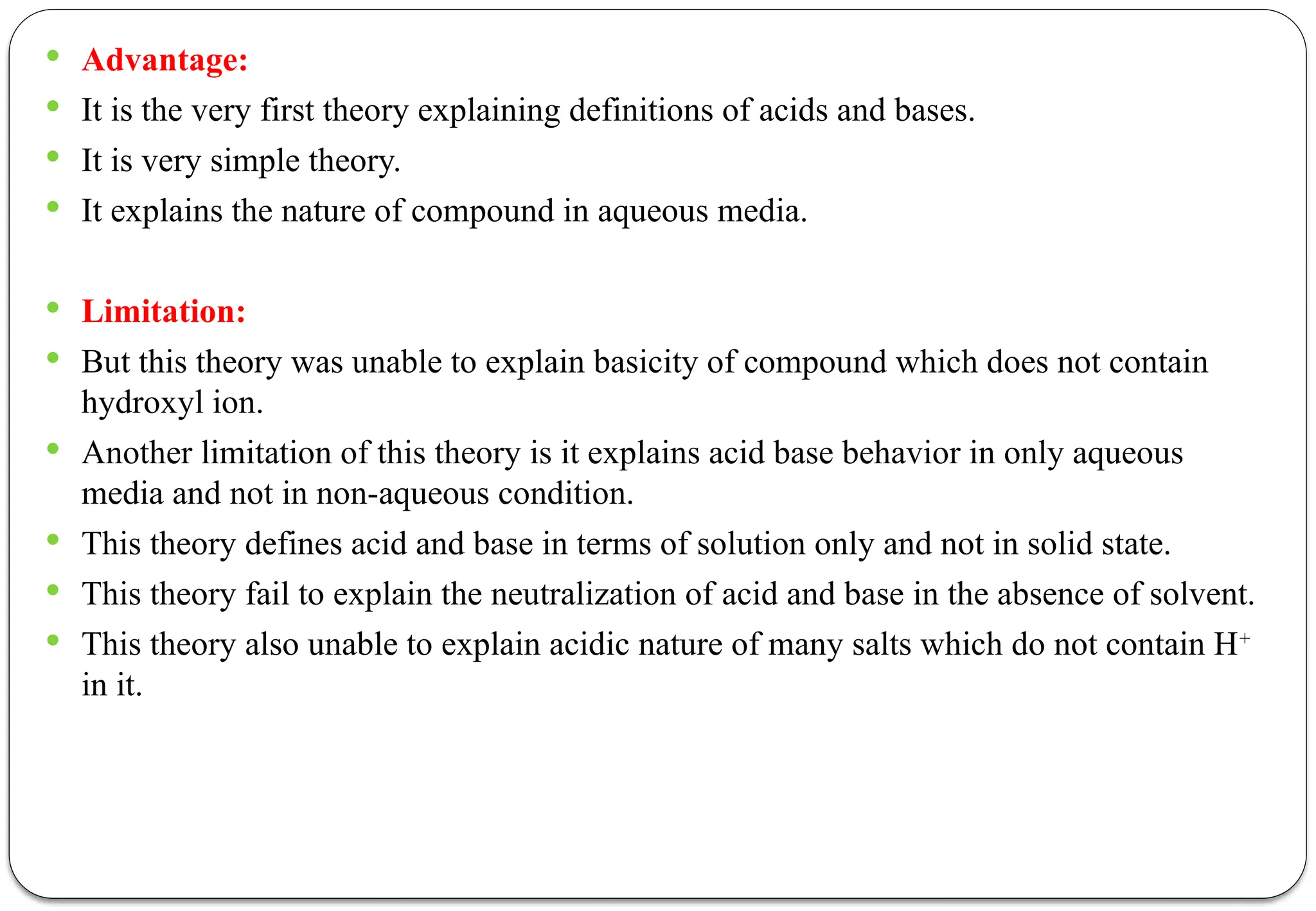
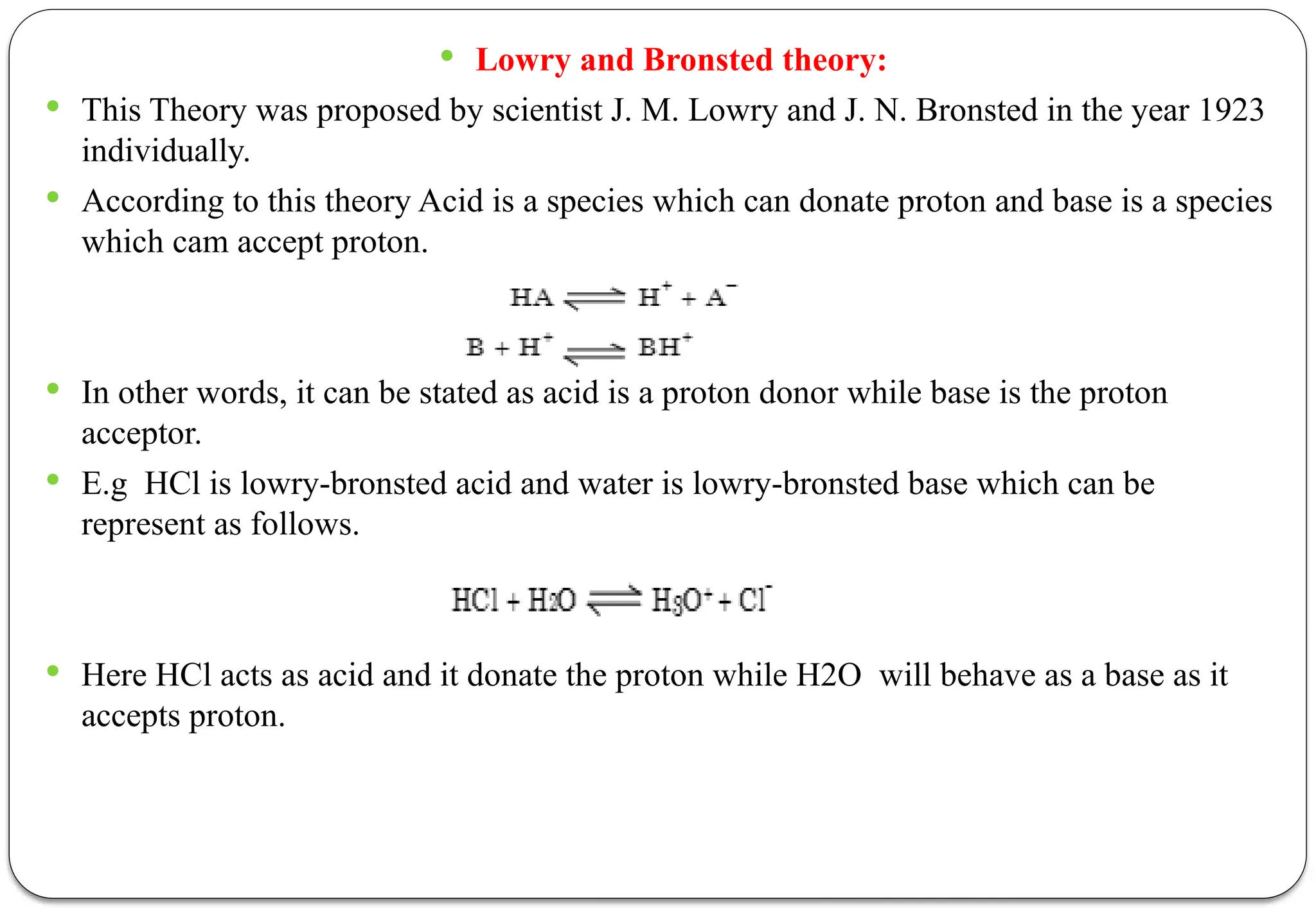
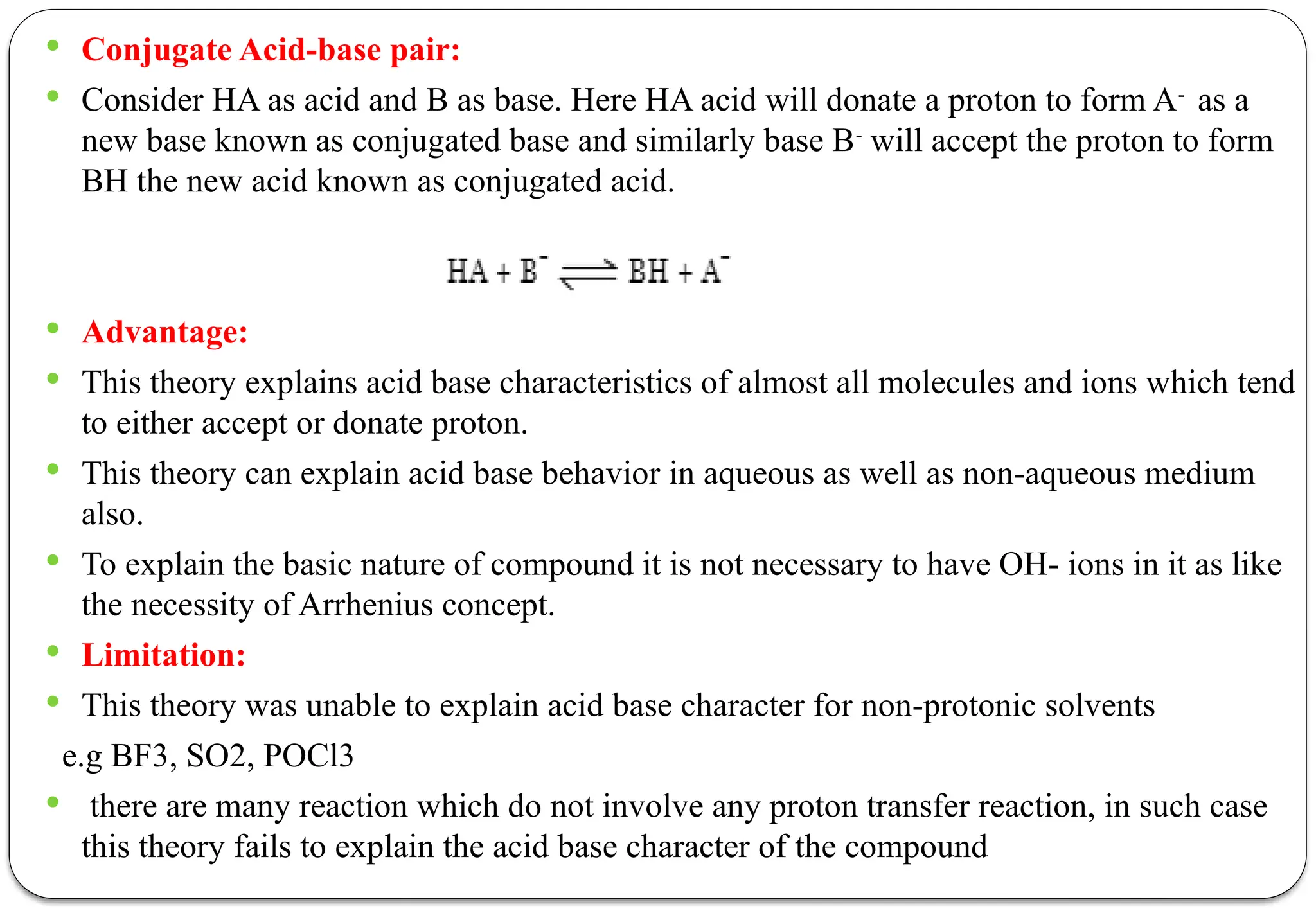
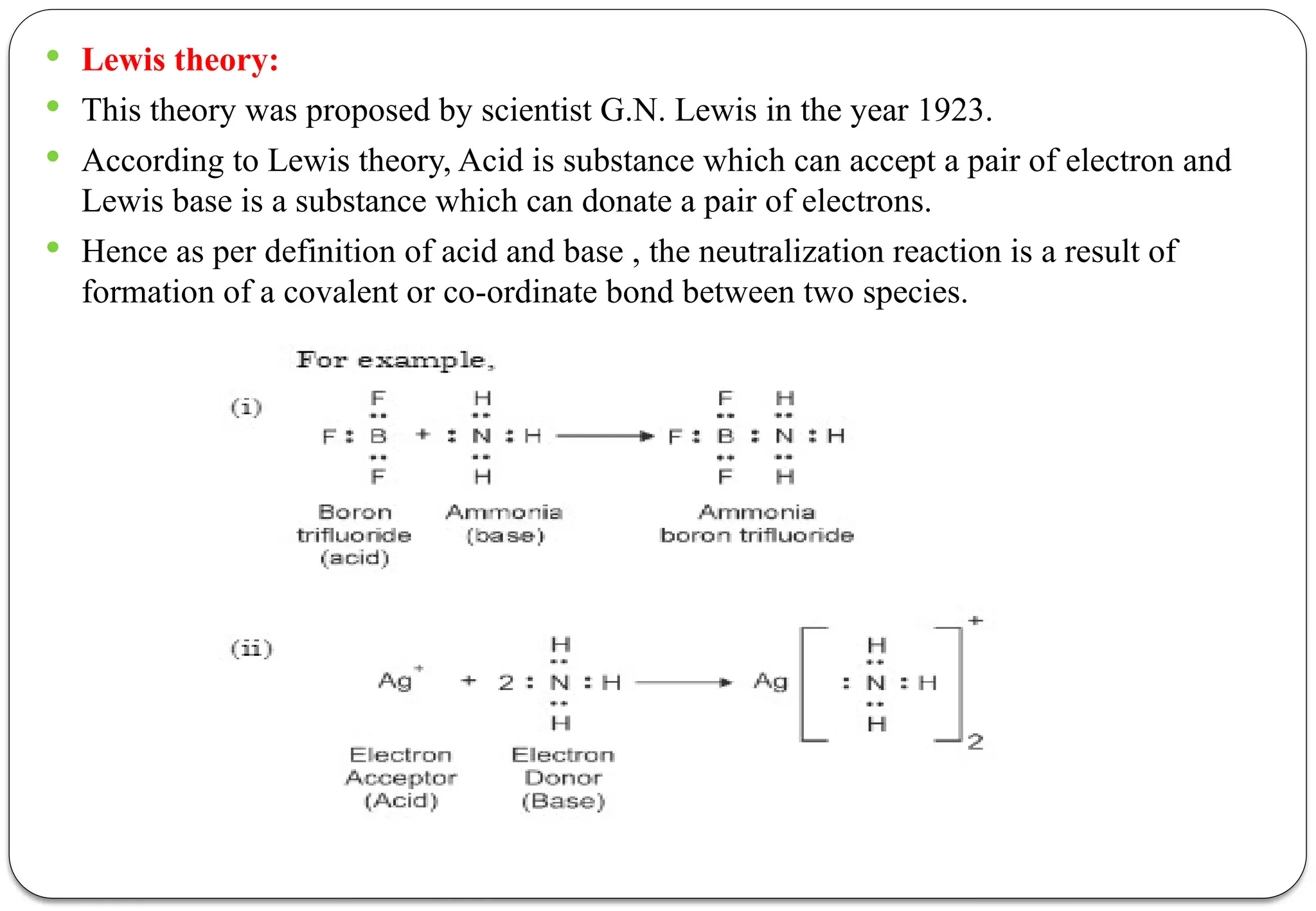
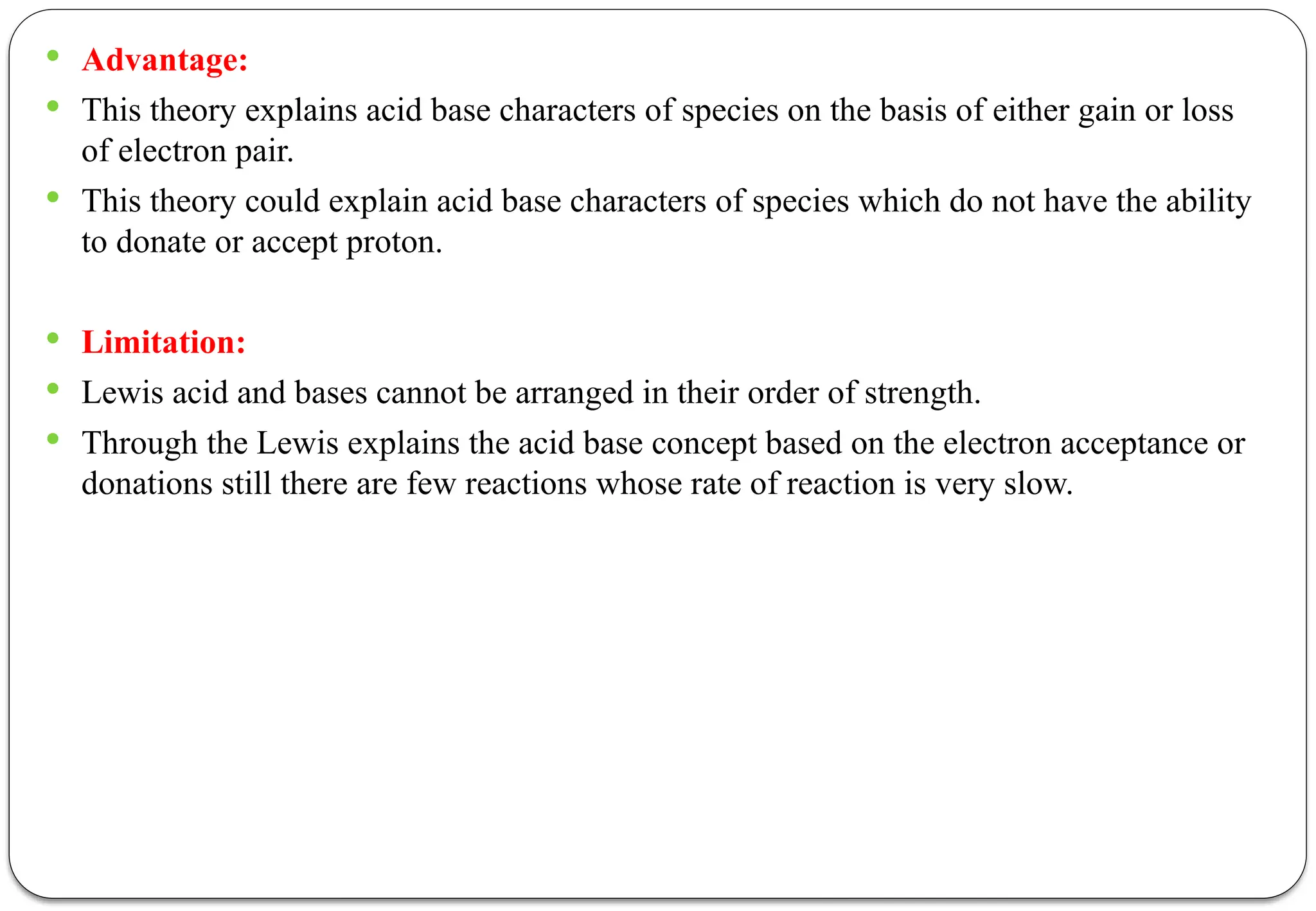
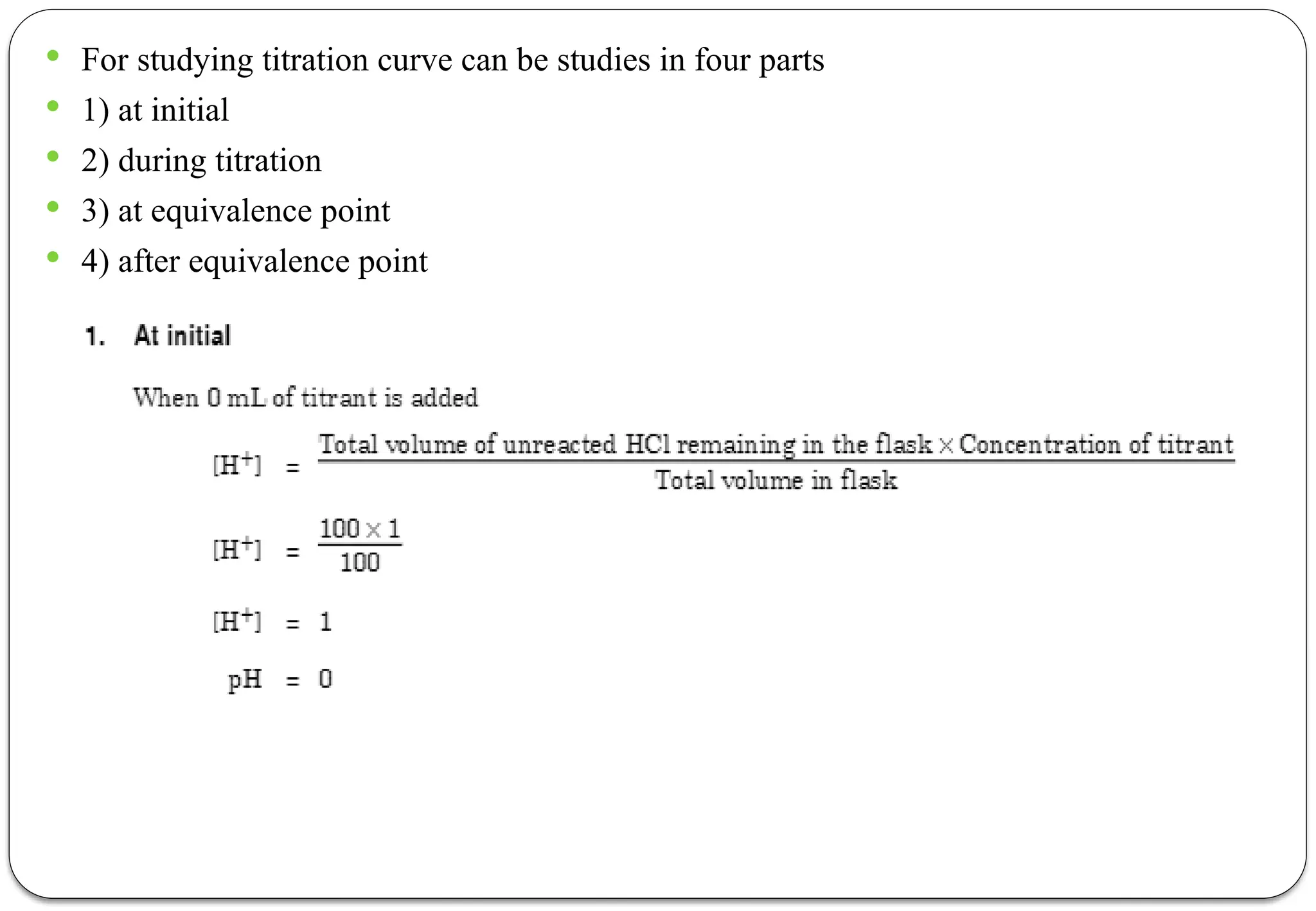
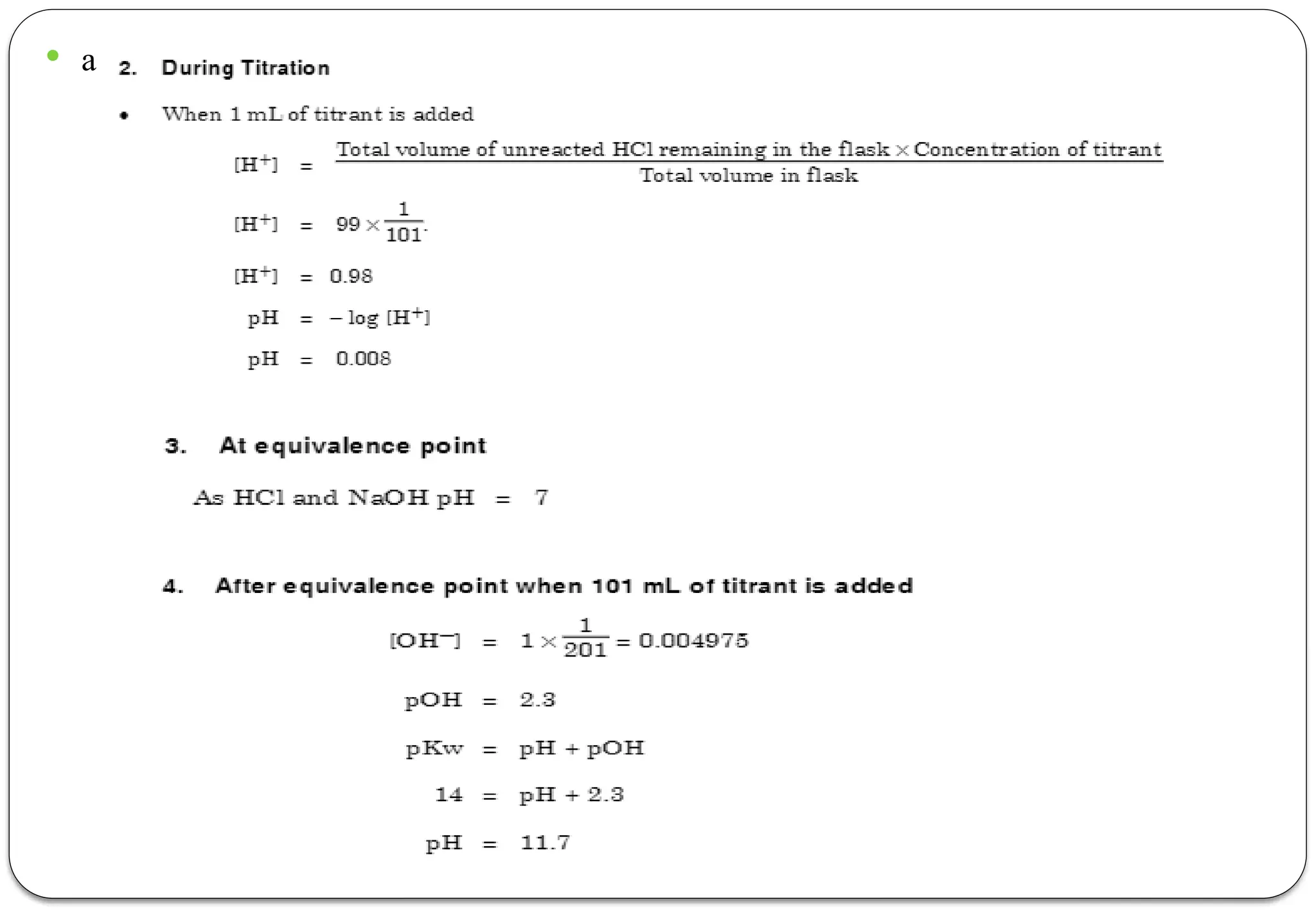
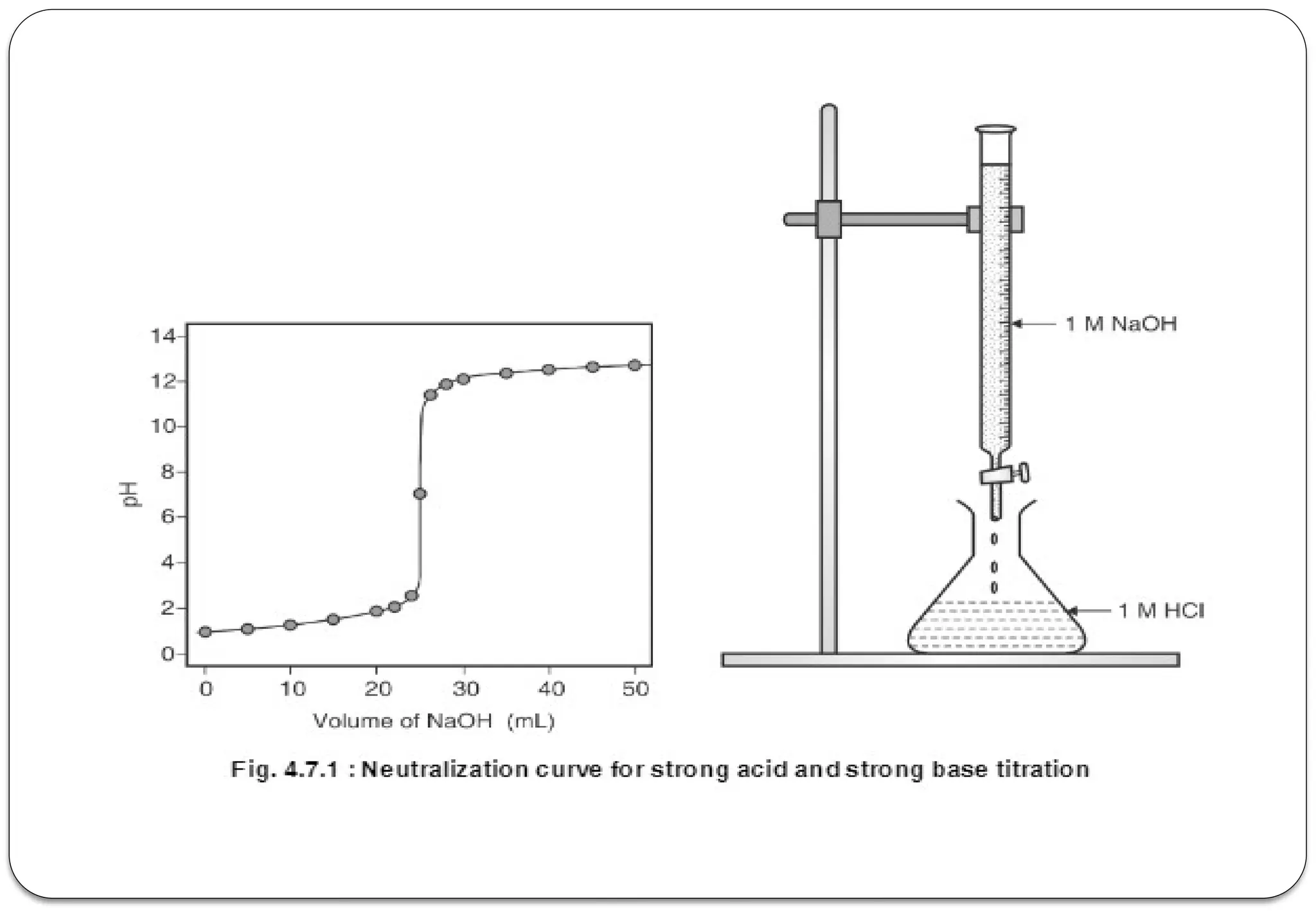
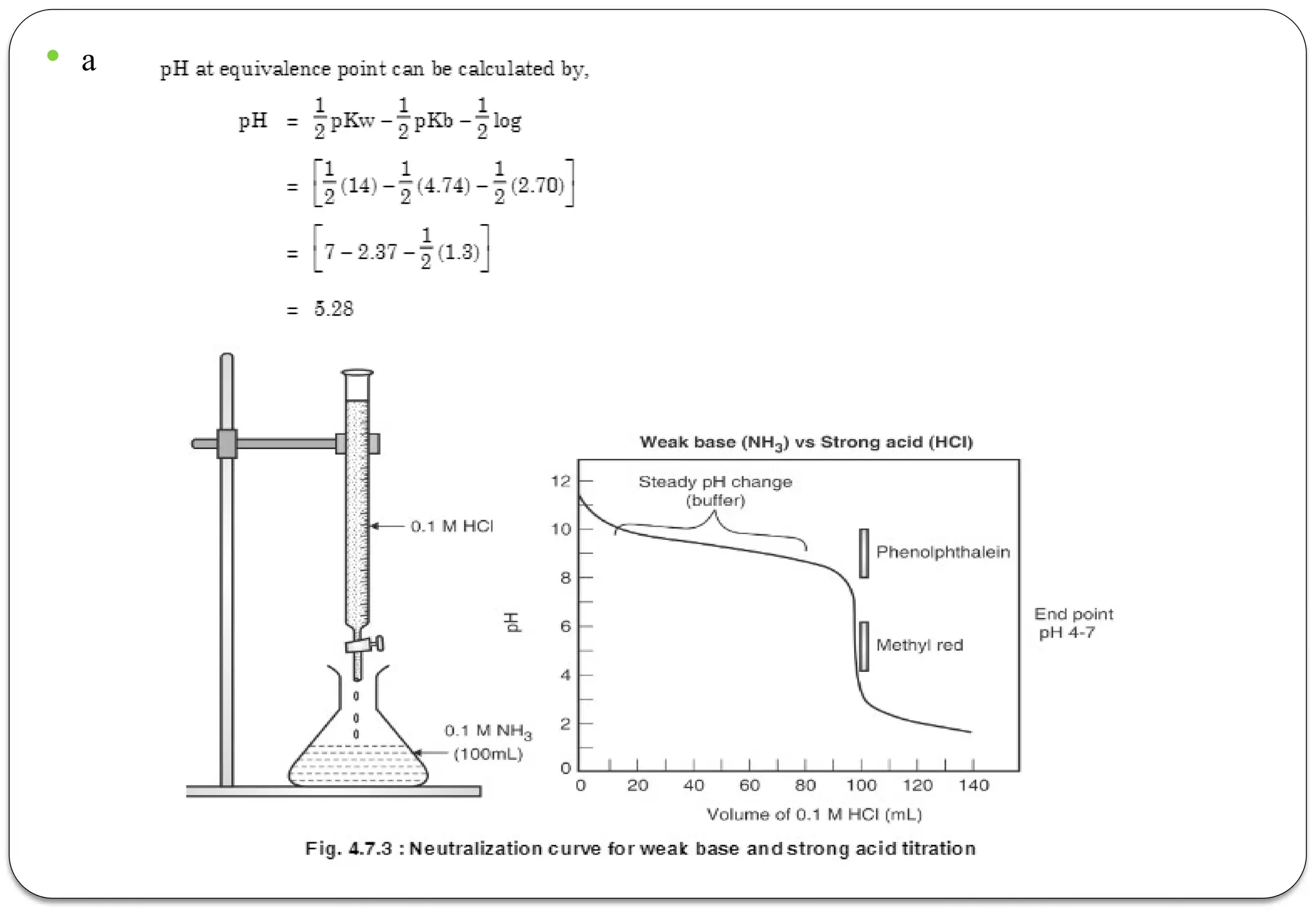
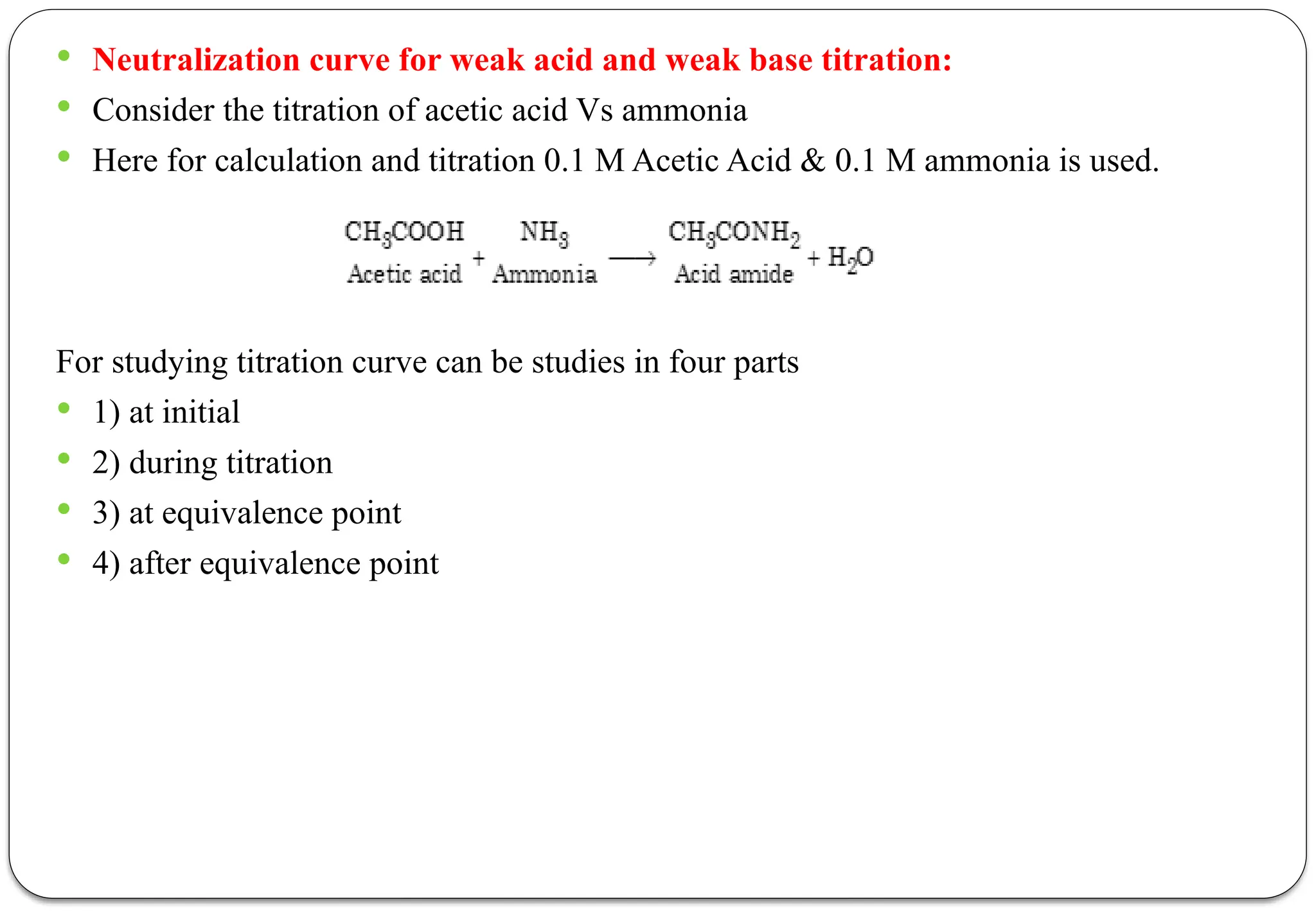
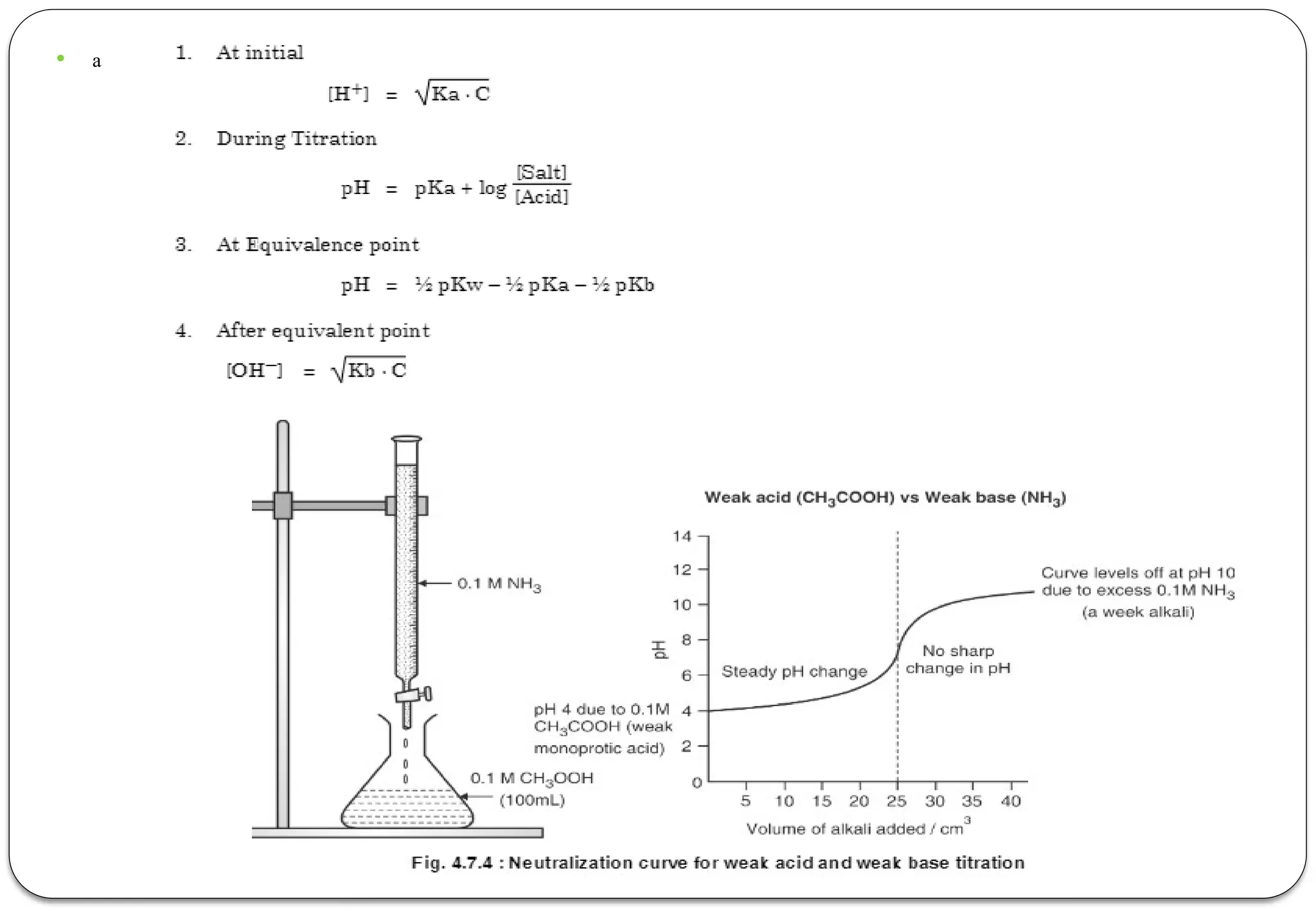
The document discusses various types of acid-base titrations, including aqueous and non-aqueous titrations, and details the theories behind acid-base behavior proposed by Arrhenius, Lowry-Bronsted, and Lewis. It explains different neutralization curves associated with strong and weak acids and bases, the role of indicators in titrations, and the characteristics of solvents used in non-aqueous titrations. Additionally, it addresses various methods for detecting end points and the advantages and limitations of each theoretical approach.



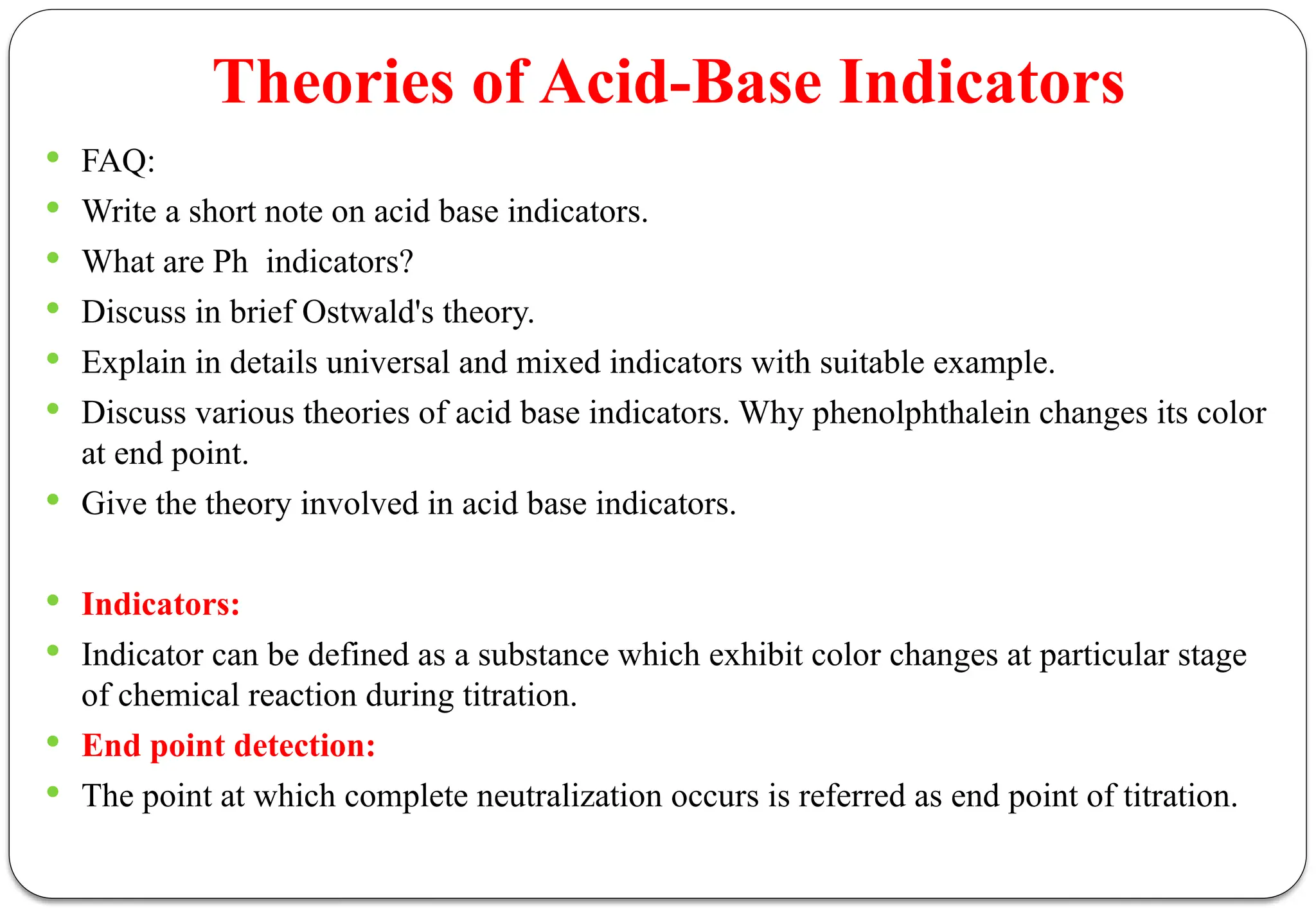

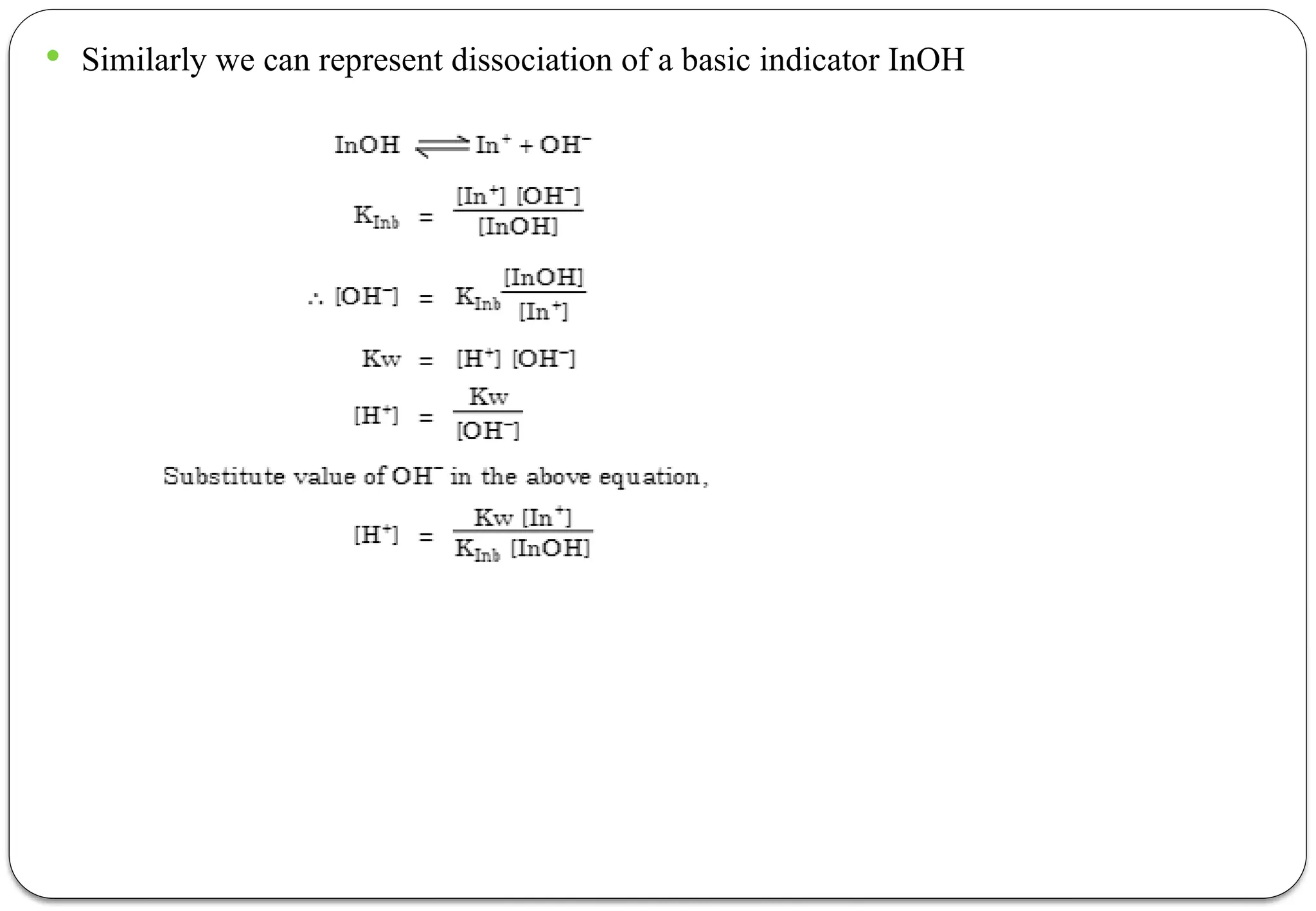
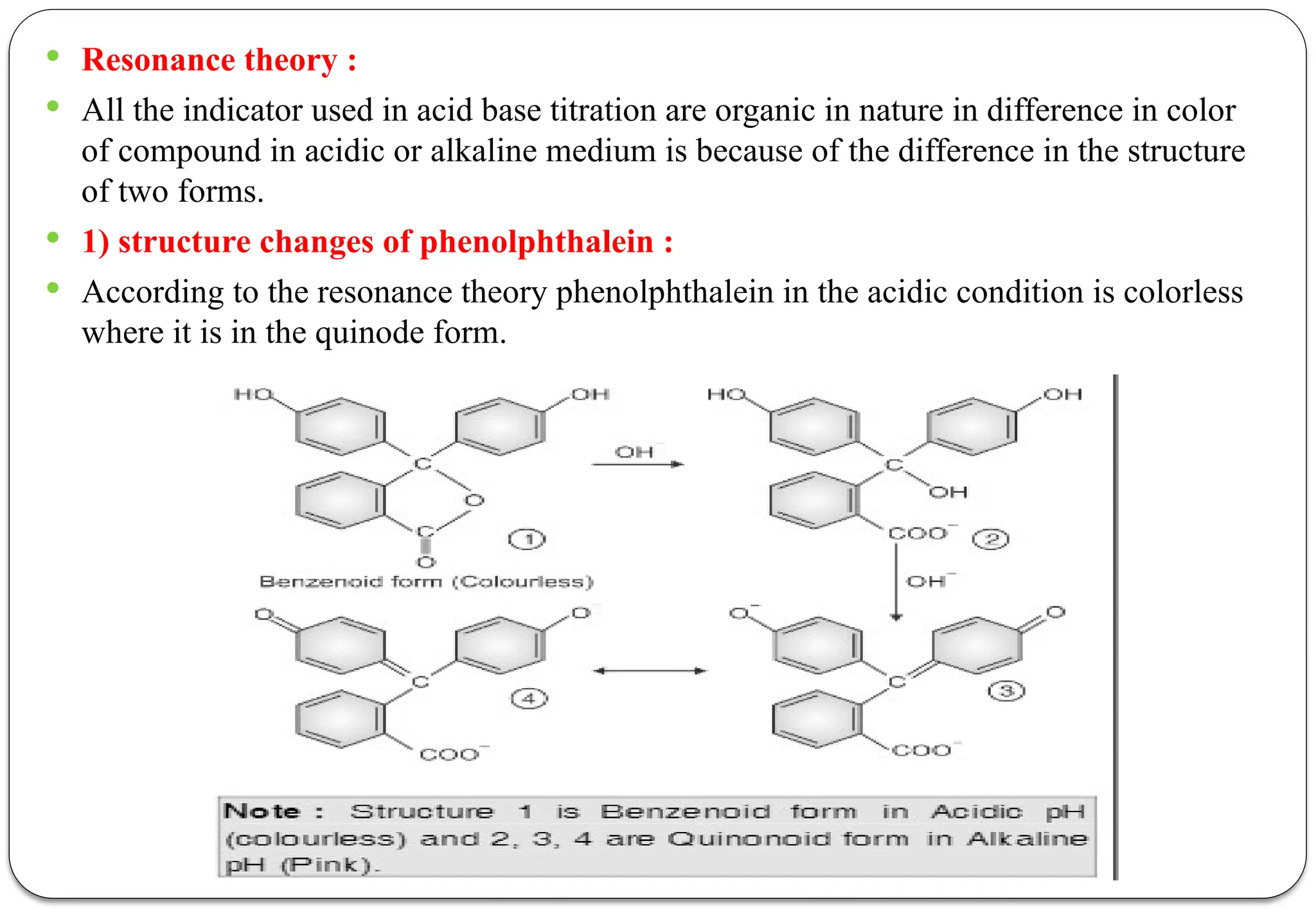
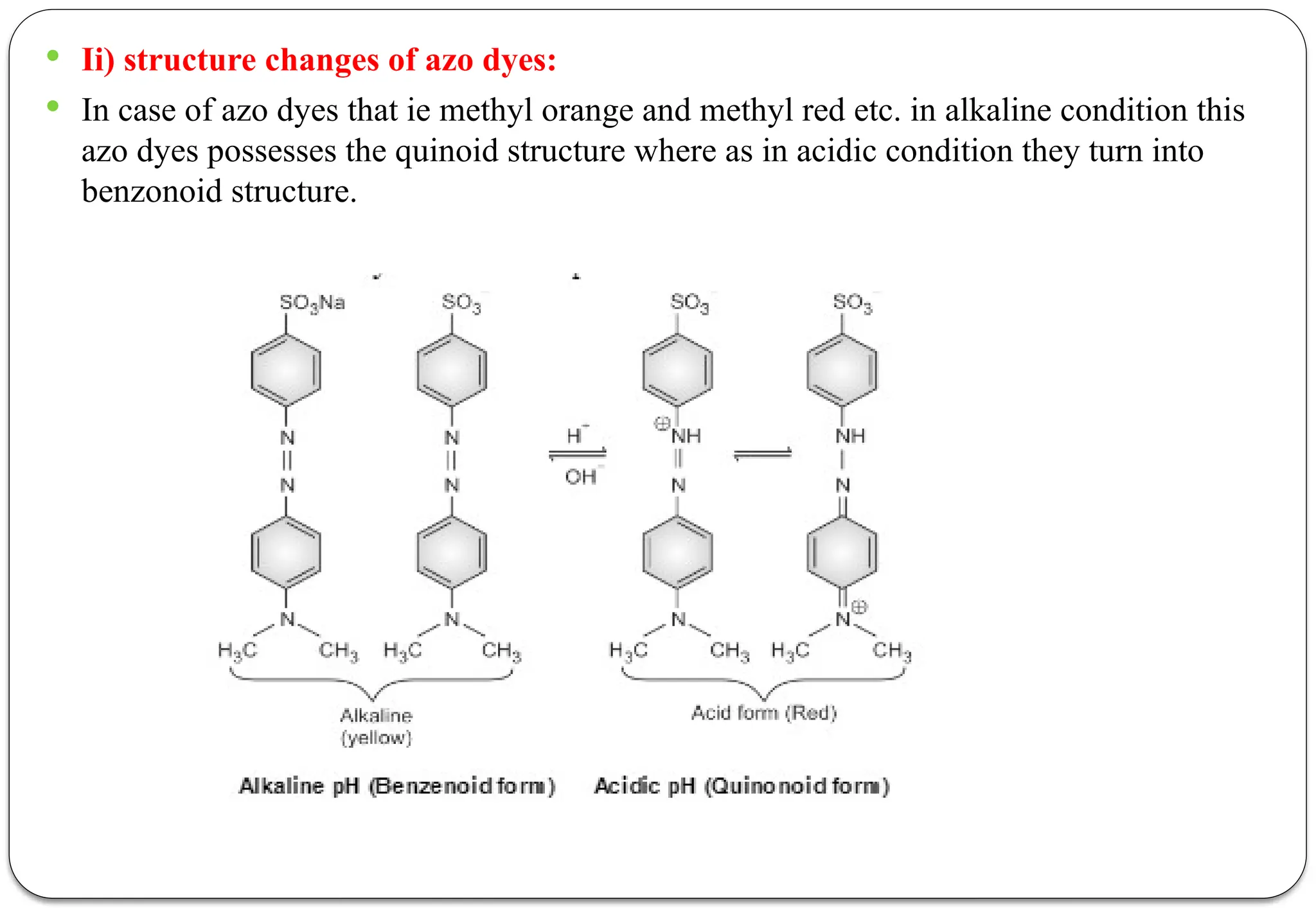
![ According to this theory the undissociated acidic indicators [HIn] and basic indicator
[InOH] has different color than that of its ions or dissociate form.
Therefore the dissociated of an acidic indicator can be written as follows.
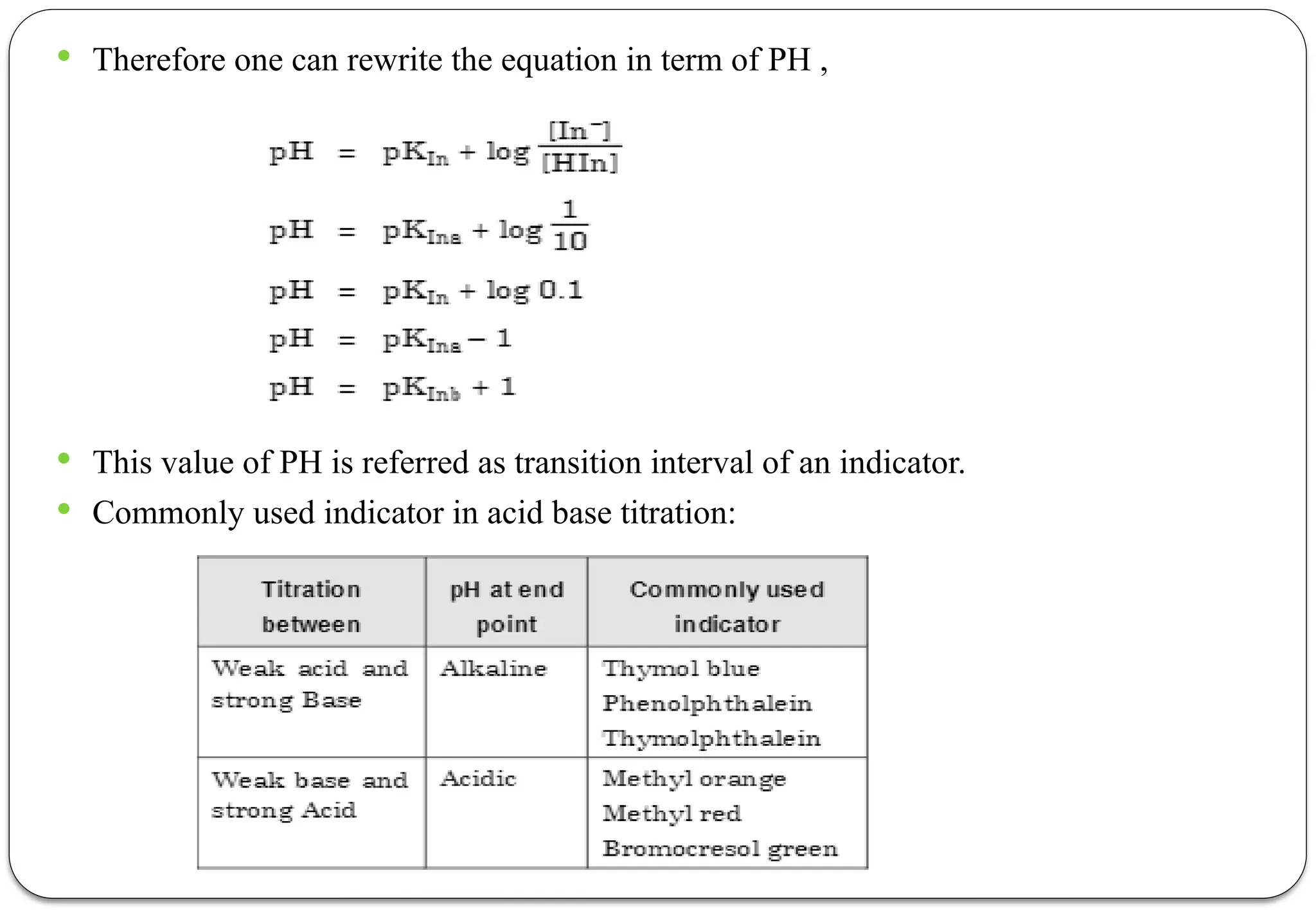
Due to this the color of solution changes which is the result of increased concentration
of ionized form of an indicator.](https://image.slidesharecdn.com/pa-unit2-250207052033-19ce080a/75/Pharmaceutical-Analysis-BP102T-UNIT-II-Acid-Base-Titration-24-2048.jpg)