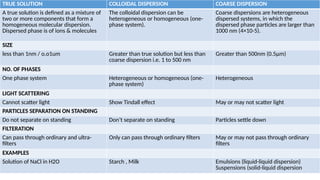
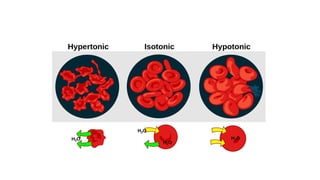
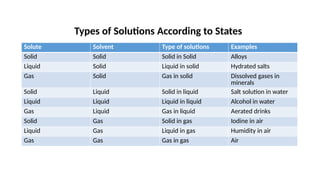
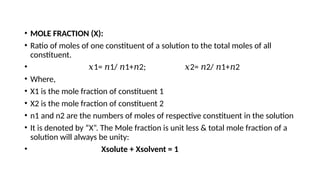
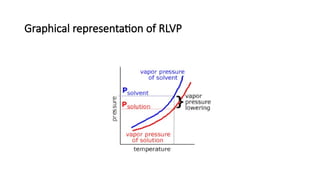
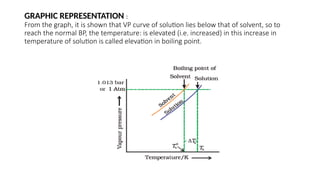
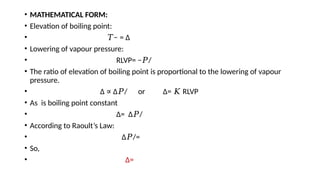
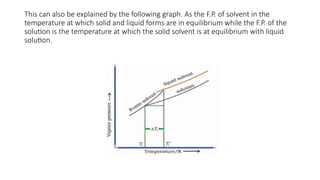
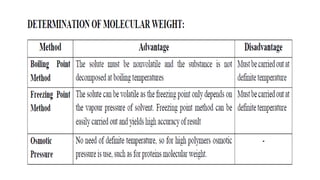
The document details the characteristics, types, and properties of solutions in physical pharmacy, explaining key concepts such as solute, solvent, and phases. It covers the definitions of various solution types like true solutions, colloidal dispersions, and coarse dispersions, including their colligative properties and concentration expressions. Additionally, it differentiates between ideal and real solutions, elaborating on Raoult's law and the effects of solute concentration on boiling and melting points.