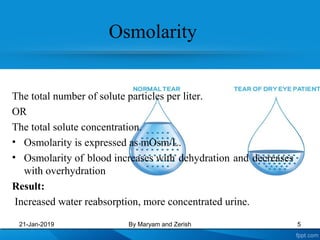
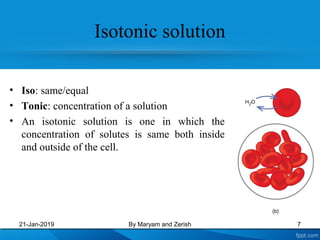
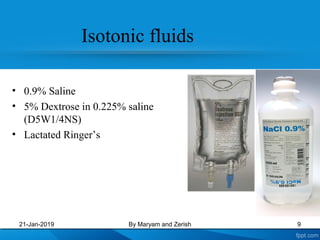
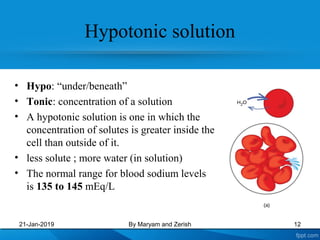
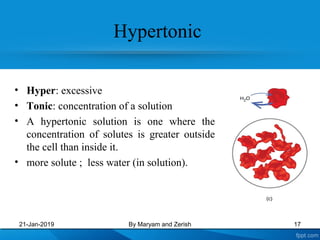
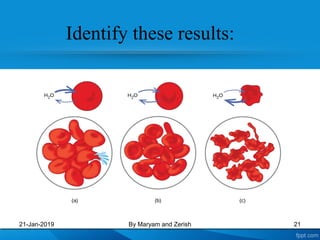
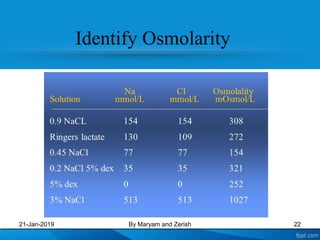
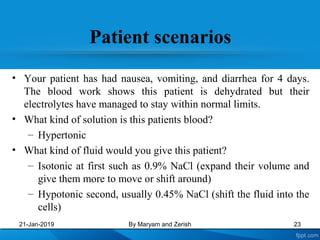
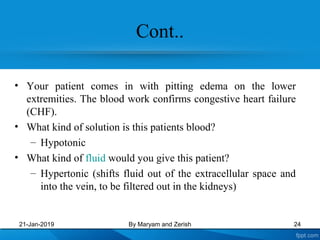
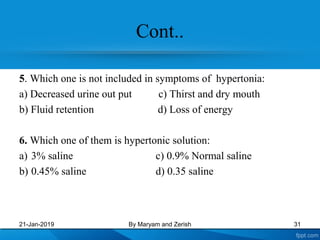
This presentation defines solutions, solvents, and solutes. It discusses isotonic, hypotonic, and hypertonic solutions, and the effects they have on cells. Isotonic solutions like 0.9% saline cause no change in cell volume. Hypotonic solutions cause water to enter cells, while hypertonic solutions cause water to leave cells. The appropriate IV fluid depends on whether the patient's blood is isotonic, hypotonic, or hypertonic. Isotonic fluids are used to expand extracellular volume, while hypotonic fluids put fluid back into cells and hypertonic fluids remove fluid from cells.