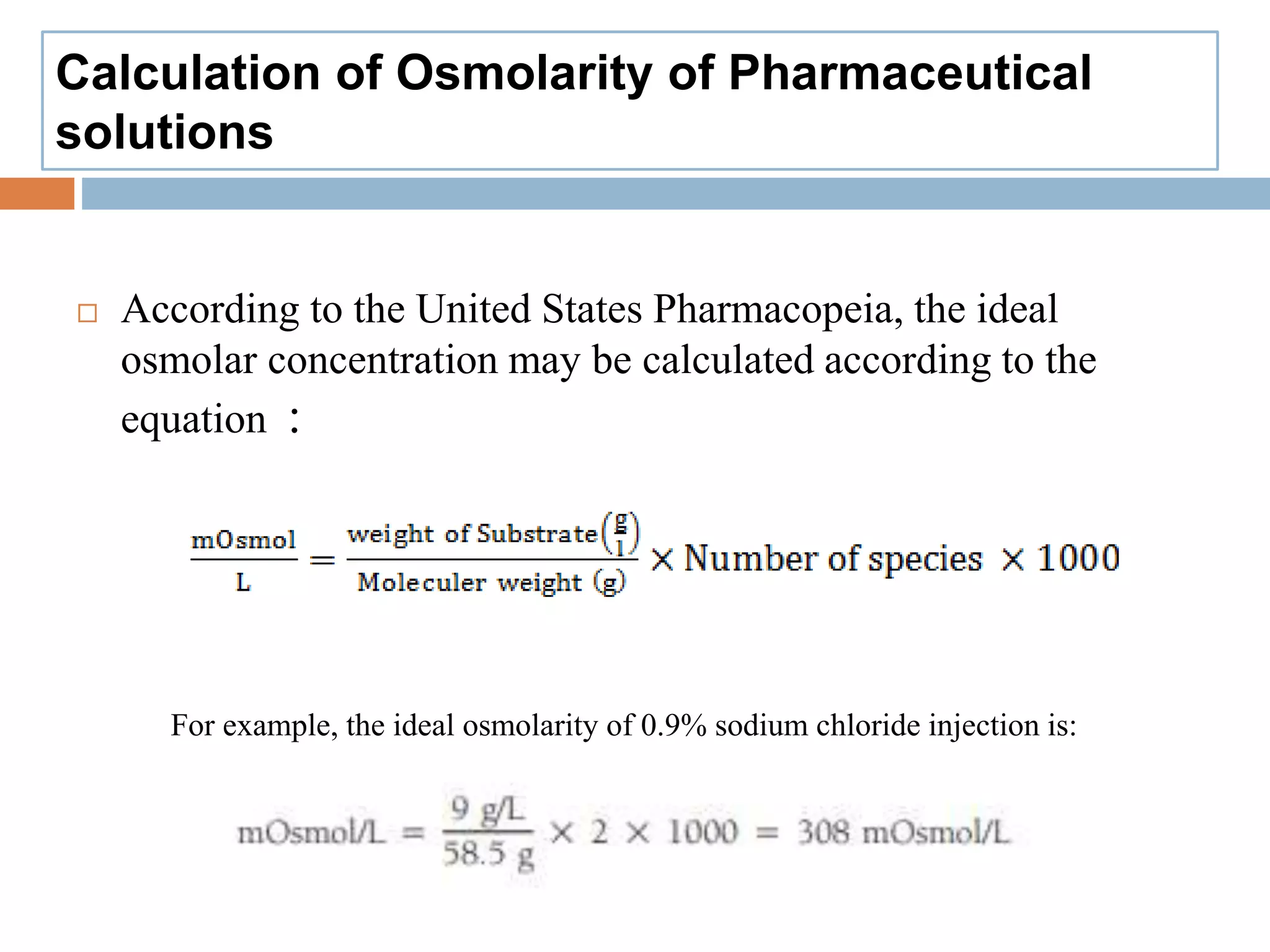
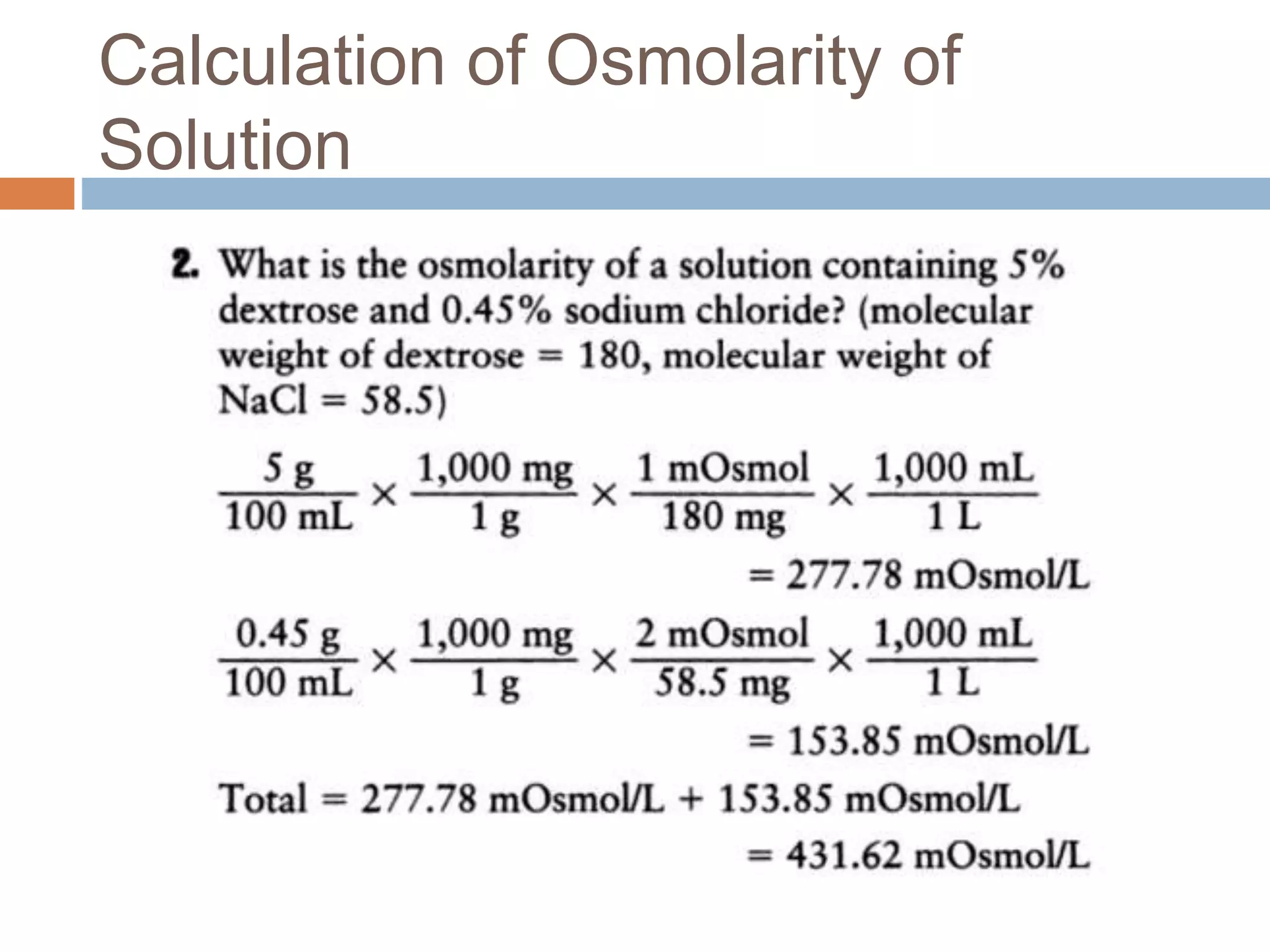
The document discusses the concepts of osmolality and osmolarity in pharmaceutical technology, explaining their significance in drug formulation and administration. It outlines the classification of solutions and the importance of isotonic solutions to prevent irritation and maximize drug efficacy. Additionally, it details the methods for calculating osmolarity and the physiological relevance of osmotic properties in medical practice.