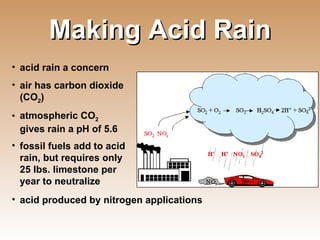
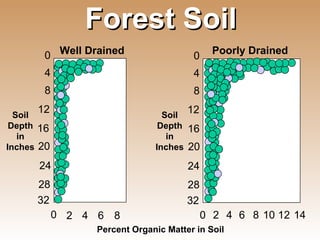
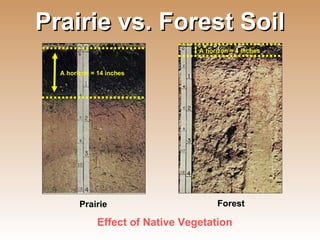
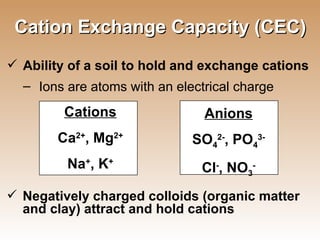
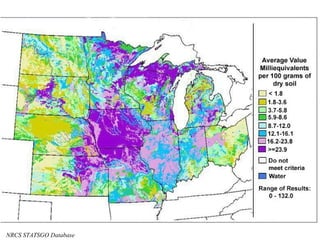
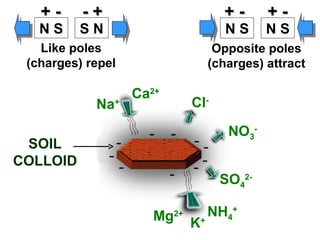
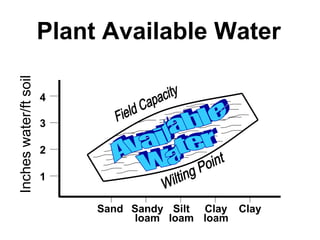
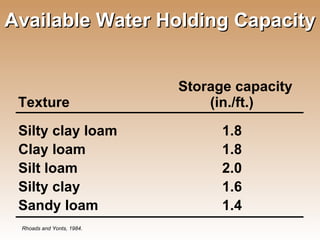
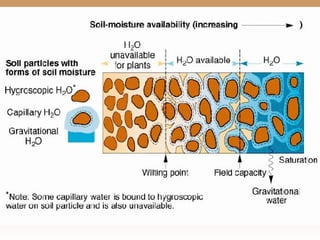
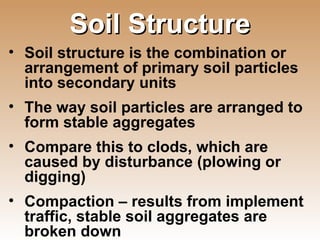
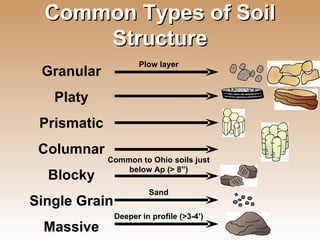
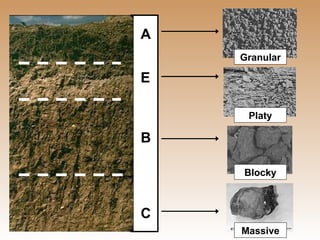
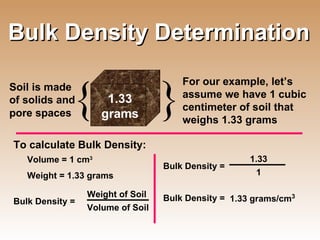
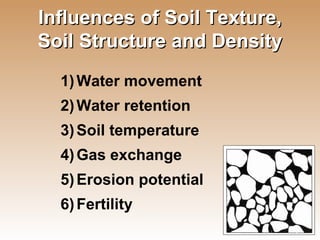
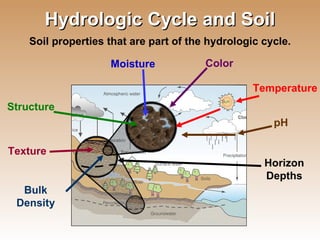
The document provides an introduction to soil, defining it and discussing its importance as a resource for plant growth, nutrient cycling, and environmental filtering. It covers various aspects such as soil color, texture, structure, pH, and cation exchange capacity, as well as factors affecting soil properties. The training aims to enhance understanding of soil management and its implications for agriculture and ecology.


























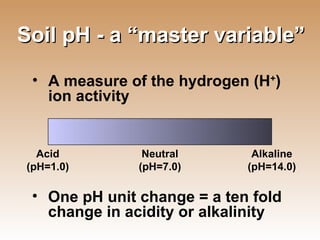


![Soil pH Methods for soil pH determination can vary widely Measure of the direct concentration of H + ions in the soil solution Buffer pH measures both H + ions in the soil solution and the reserve H + ions bound on cation exchange sites It is used to express the acidity or alkalinity of the soil solution, not lime requirement pH represents the equation -log[H + ]](https://image.slidesharecdn.com/021009soilintro-1232562145566524-3/85/Introduction-to-Soil-Science-39-320.jpg)