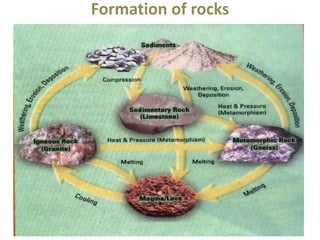
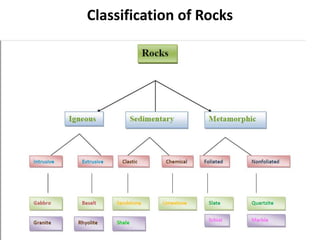
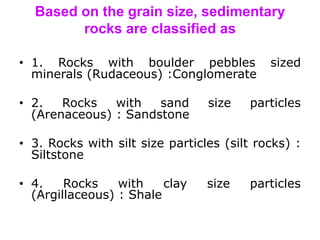
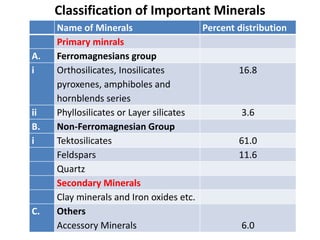
This document discusses the classification of rocks and minerals. It describes three main types of rocks: igneous, sedimentary, and metamorphic. Igneous rocks form from the cooling of magma, sedimentary rocks form through the accumulation and cementation of sediments, and metamorphic rocks form from alterations to existing rocks by heat, pressure, and chemically active fluids. Within each rock type are various sub-classifications. The document also examines the classification of important rock-forming minerals and describes their structures, weathering properties, and physical characteristics.