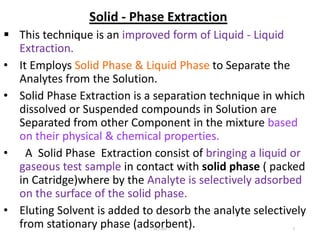
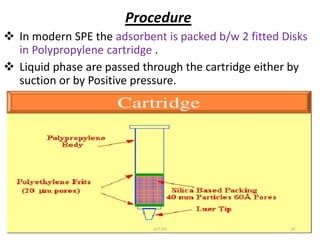
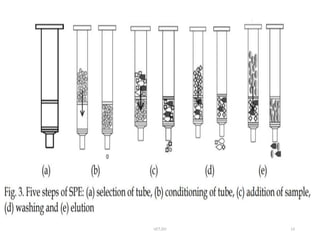
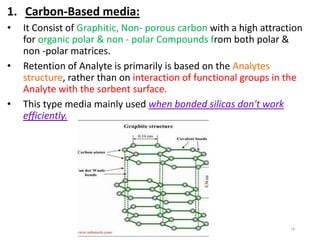
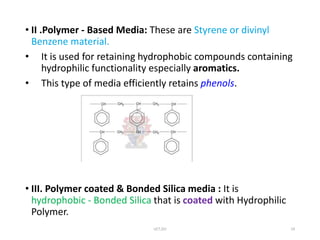
This document discusses solid-phase extraction, an improved form of liquid-liquid extraction used to separate analytes from biological matrices. Solid-phase extraction uses a solid stationary phase and liquid mobile phase to separate analytes based on physical and chemical properties. The procedure involves loading a sample onto a cartridge containing a solid stationary phase, washing away contaminants, and then eluting the analyte for analysis. Common mechanisms for retention include normal phase, reverse phase, and ion exchange interactions. Solid-phase extraction has advantages over liquid-liquid extraction like lower solvent use and easier analyte collection.