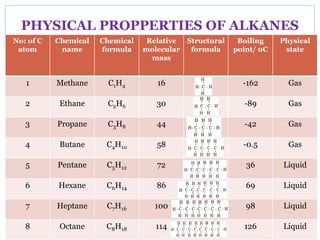
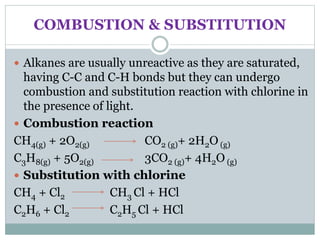
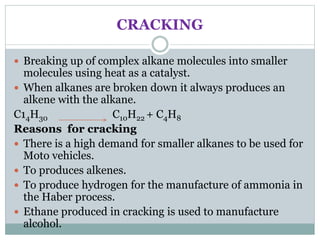
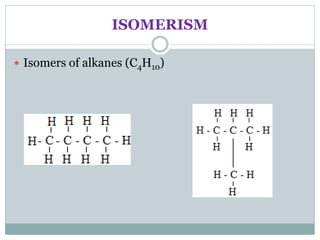
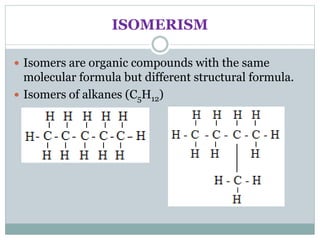
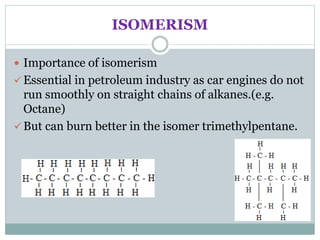
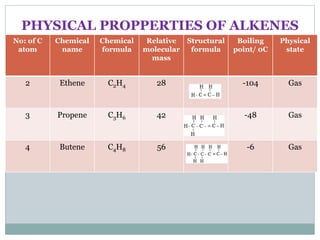
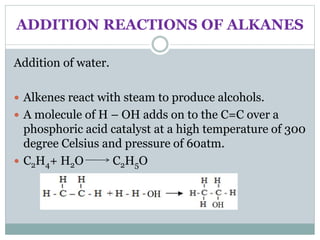
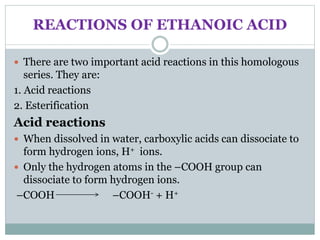
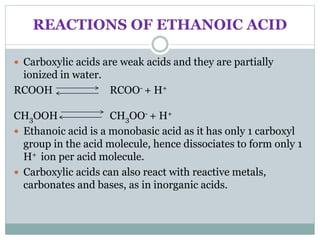
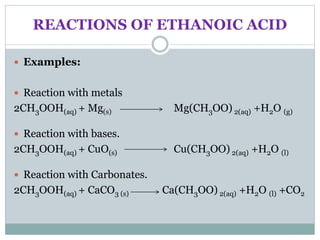
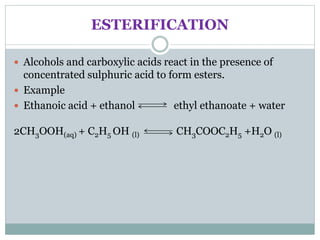
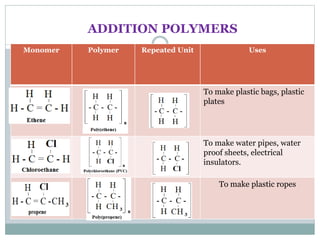
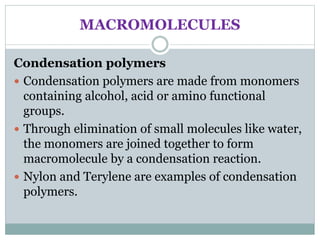
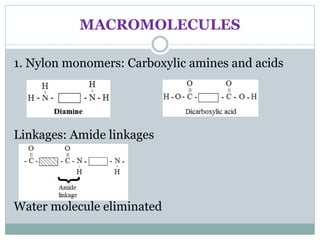
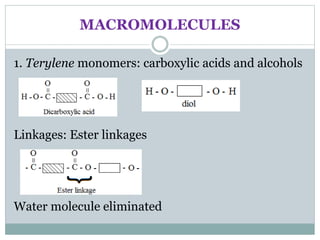
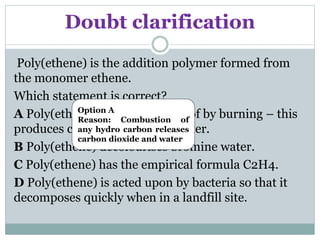
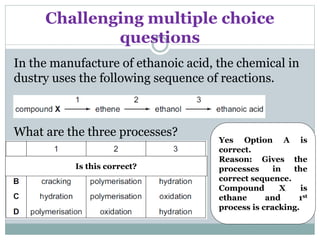
This document provides an overview of organic chemistry topics including alkanes, alkenes, alcohols, carboxylic acids, and macromolecules. It defines key terms such as homologous series and discusses the physical properties and reactions of these organic compounds. For example, it explains that alkanes form a homologous series with a general formula of CnH2n+2 and that their melting and boiling points increase with chain length. It also summarizes how alcohols can undergo combustion, oxidation to form carboxylic acids, and esterification reactions.









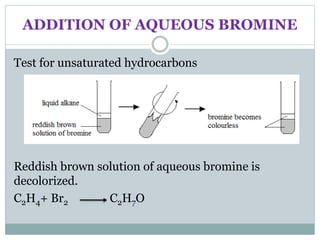
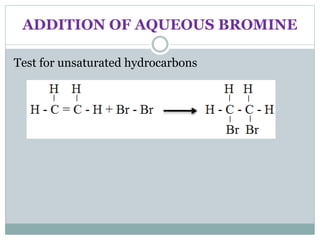





![REACTION OF ALCOHOLS
Oxidation
Alcohols can undergo oxidation to form carboxylic
acids.
In this reaction alcohol will lose two hydrogen atoms and
gain 1 oxygen atom.
C2H5OH(l) + 2[O] (g) CH3COOH(l) + H2O(l)](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-28-320.jpg)


![FORMATION OF CARBOXYLIC ACIDS
Formation of ethanoic acid by oxidation of ethanol.
1. Using atmospheric oxygen.
C2H5OH(l) +O2(g) CH3COOH(l) + H2O(l)
2. Using acidified potassium dichromate.
C2H5OH(l) +2[O] (g) CH3COOH(l) + H2O(l)
The orange potassium dichromate (VI) solution changes to
green.](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-32-320.jpg)


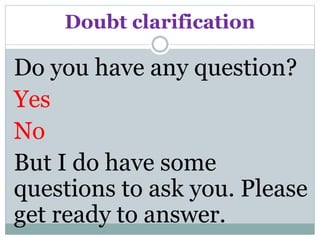
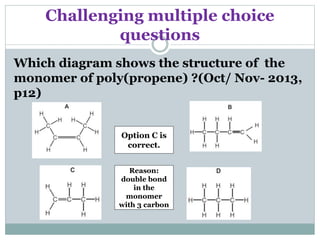



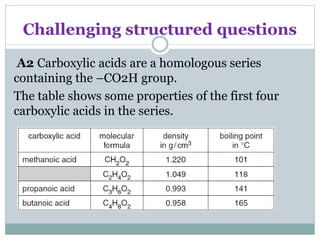
![Challenging structured questions
(a) (i) Describe how the density of these carboxylic
acids varies with the number of
carbon atoms in the molecule.
....................................................................................[1]
(ii) Name the carboxylic acid with the molecular
formula C2H4O2.
................................................................................... [1]
(iii) Draw the structure of propanoic acid, showing
all atoms and bonds. [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-54-320.jpg)
![Challenging structured questions
(b) The next carboxylic acid in this homologous series is pentanoic
acid.
Pentanoic acid has five carbon atoms.
(i) Deduce the molecular formula for pentanoic acid.
................................................................................... [1]
(ii) Suggest a value for the boiling point of pentanoic acid.
.............................................°C [1]
(c) Butanoic acid, C3H7CO2H, reacts with sodium to form a salt
and a gas.
(i) Name the gas.
........................................................................................................... [1]
(ii) Give the formula of the salt.
............................................................................................................[1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-55-320.jpg)
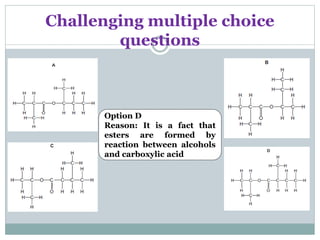
![Challenging structured questions
(d) Esters are formed when carboxylic acids react with
alcohols.
The reaction is catalysed by hydrogen ions.
(i) Describe and explain the effect of a catalyst on
reaction rate.
..............................................................................................
..............................................................................................
......................................................................................... [2]
(ii) State one commercial use of esters.
......................................................................................... [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-56-320.jpg)
![Challenging structured questions
(iii) The structure of an ester is shown below.
Name this ester.
................................................................................... [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-57-320.jpg)
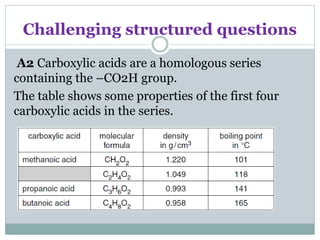
![Challenging structured questions
(a) (i) Describe how the density of these carboxylic acids
varies with the number of
carbon atoms in the molecule.
decreases as number of carbon atoms increases / increases as
number of carbon atoms decreases [1]
(ii) Name the carboxylic acid with the molecular formula
C2H4O2. [1]
Ethanoic acid
(iii) Draw the structure of propanoic acid, showing all atoms
and bonds. [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-59-320.jpg)
![Challenging structured questions
(b) The next carboxylic acid in this homologous series is
pentanoic acid. Pentanoic acid has five carbon atoms.
(i) Deduce the molecular formula for pentanoic acid.
[1]
(ii) Suggest a value for the boiling point of pentanoic acid.
180 - 190°C [1]
(c) Butanoic acid, C3H7CO2H, reacts with sodium to form a
salt and a gas.
(i) Name the gas.
Hydrogen [1]
(ii) Give the formula of the salt.
C3H7CO2Na/ C4H7O2Na [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-60-320.jpg)
![Challenging structured questions
(d) Esters are formed when carboxylic acids react
with alcohols.
The reaction is catalysed by hydrogen ions.
(i) Describe and explain the effect of a catalyst on
reaction rate.
speeds up reaction (rate) / reaction faster/ lowers
activation energy/ [2]
(ii) State one commercial use of esters.
solvent / fragrance / perfume / food additive /
flavourings / polyesters / terylene [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-61-320.jpg)
![Challenging structured questions
(iii) The structure of an ester is shown below.
Name this ester.
propyl methanoate [1]](https://image.slidesharecdn.com/organicchemistry-160518060738/85/Organic-chemistry-62-320.jpg)