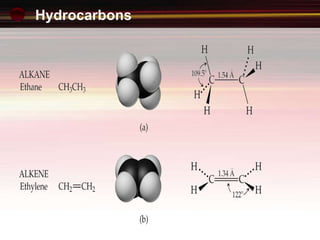
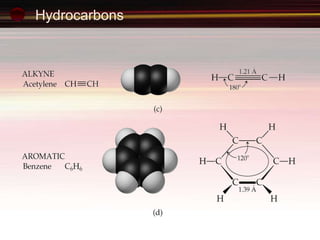
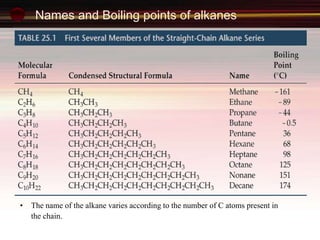
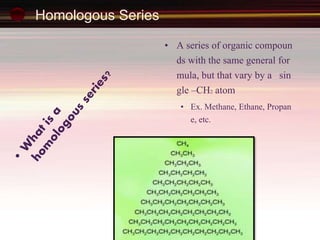
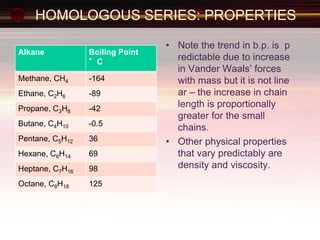
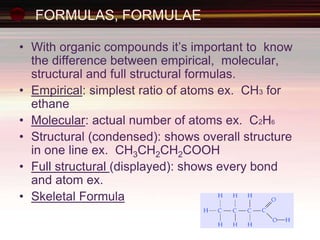
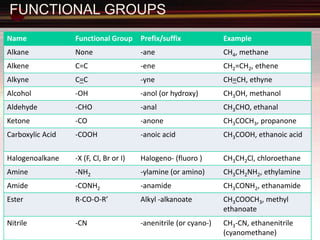
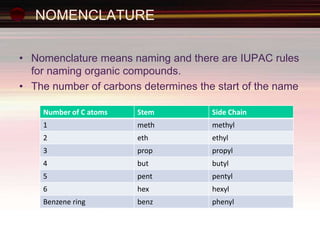
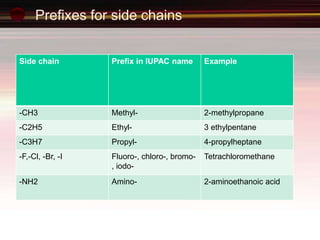
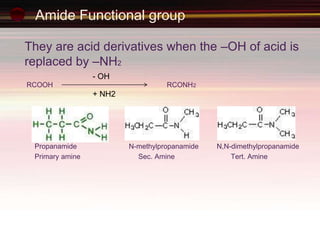
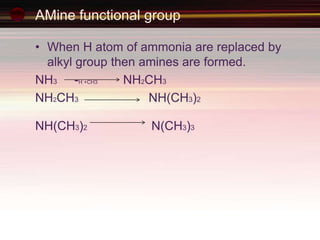
This document provides an introduction to organic chemistry, focusing on hydrocarbons. It defines organic chemistry as the study of carbon-based compounds, particularly hydrocarbons rather than metal carbonates or oxides. There are four main classes of hydrocarbons discussed: alkanes, alkenes, alkynes, and aromatics. Alkanes contain only single bonds, while alkenes contain double bonds and alkynes contain triple bonds. Functional groups are discussed, which determine the physical and chemical properties of organic compounds. Homologous series are introduced as families of compounds that differ by CH2 units and have similar properties.